Abstract
Harpins are type three secretion system (TTSS) effectors. While few harpins are thought to be translocators of TTSS effectors through the host plasma membrane during plant/bacteria interactions, functions of many harpins remain for the moment mysterious. We recently showed that the HrpWea harpin from Erwinia amylovora, at subnamolar concentration, was able to decrease defense responses triggered by another harpin from this bacteria, HrpNea. This antagonism could be the result of opposed anion channels modulations triggered by HrpWea and HrpNea. At upper concentrations HrpWea alone, or in combination with HrpNea, was able to induce cell death. This form of cell death involves strong ion channel activation and shares similarity with apoptosis volume decrease (AVD), a form of programmed cell death well described in animal cells. All these results suggest different ways for harpins to trigger cell death and highlight the role of ion channels during cell death processes.
The type III secretion system (TTSS), which is present in most plant and animal pathogenic Gram-negative bacteria, is determinant for many interactions since it allows delivery of specific bacterial proteins to host cell.Citation1 Bacterial TTSS effectors are highly diverse and collectively manipulate host cell metabolism to improve bacterial nutrition or to encounter cell defense mechanisms. Among TTSS effectors, harpins constitute a unique family of TTSS effectors that are not found in mammalian pathogens. While their function still remains unclear during bacteria / host plants interactions, harpins are supposed to be able to trigger HR cell death and associated defense responses when infiltrated in nonhost plants.Citation1
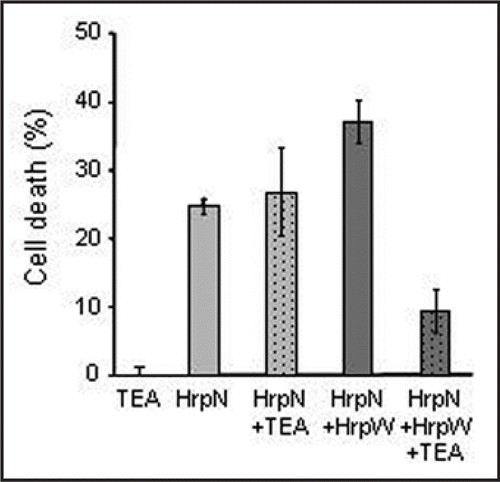
We recently showed by using Erwinia amylovora mutant strains and purified proteins that the HrpWea harpin, at subnanomolar concentrations could antagonize cell death and AOS production induced by HrpNea, another harpin, secreted by Erwinia amylovora.Citation2 Our results showed that this antagonism is probably the result of opposed ion channels modulations triggered by HrpWea and HrpNea ( and 1B) and allowed us to hypothesize that HrpWea could be important for E. amylovora initial survival in nonhost plants by decreasing plant cell defenses.Citation2 In animal system, YopB, a TTSS translocator of Yersinia, is important for pro-inflammatory signaling response in epithelial cells infected with Y. pseudotuberculosis.Citation3 YopB could stimulate signaling through a cytoplasmic domain following insertion into the membrane or through an interaction with a receptor in the membrane. However, three Yop effectors, YopE, YopH, and YopJ, counteracted this response in infected cells. It is speculated that YopB signaling induces changes in the cell that are required for an efficient translocation process and that once proper amounts of Yops are translocated, the process would be shut down to avoid further cell damage caused by excessive signaling. In the same way, we could speculate that signaling induced by HrpNea, a putative translocator of E. amylovora TTSS effectors through the host plasma membrane,Citation4,Citation5 which posses a cytoplasmic domain which harbors a protein binding site,Citation6 is shut down by subnanomolar concentrations of HrpWea, allowing a decrease of basal immunity to prolong colonization. Although phenotype of a hrpWea mutant strain indicates that HrpWea likely acts as a cell death inhibitor when secreted by E. amylovora,Citation2 HrpWea, at higher concentrations, was also reported to induce hypersensitive response (HR) on tobaccoCitation7,Citation8 and a cell death concomitant with strong anion channels activation on A. thaliana cells.Citation2 This strong activation of anion channels could be responsible for an anion efflux and the cell death triggered by HrpWea could be similar to cell death activated in tobacco by the fungal elicitor cryptogein. In this last model, cryptogein induced cell death via anion channel mediated nitrate efflux.Citation9 In addition to nitrate efflux, cryptogein induces K+ effluxCitation10 via activation of K+ outward rectifying channels (KORC, unpublished data). The cell death triggered by cryptogein could thus be provoked by a mechanism similar to apoptosis volume decrease (AVD) described in animal cells.Citation11 According to this model, PCD could be induced by simultaneous activation of KORC and anion channels in response to an apoptosis inducer. This increase in ion conductance leads to a strong cell volume decrease and activation of a caspase,Citation11 which could be reduced by anion or K+ channel inhibitors. Pharmacological blocking of anion efflux prevents the cryptogein-induced cell death and the accumulation of transcripts encoding vacuolar-processing enzymes, a family of proteases showing caspase-1 activity and previously reported to contribute to the disruption of vacuole integrity observed during the HR.Citation9 Such experiments were unsuccessful in our model due to lethal effect of anion channel blockersCitation12 (unpublished data), in contrary of what was observed for tobacco cells.Citation13 However, simultaneous treatment of A. thaliana suspension cells with HrpNea and HrpWea at 200 nM induced a strong KORC and anion channel activation () that could be responsible for large electrolyte leakage, similarly to what was observed during cryptogein treatment in plant cells or during AVD in animal cells. This treatment also induces a stronger cell death than cell death observed in response to 200 nM HrpNea or 200 nM HrpWea alone.Citation2 We thus tested whether the ion channel activations observed could be involved in cell death triggered by simultaneous addition of HrpNea and HrpWea. Yet, anion channels blockers were not able to decrease this cell death as previously described for cell death triggered by HrpWea alone. We also tested tetraethyl ammonium (TEA), a K+ channel inhibitor known to decrease AVD. Five millimolar TEA alone did not modify A. thaliana cells survival, but decreased cell death triggered by simultaneous addition of HrpNea and HrpWea 200 nM (). It is noteworthy that TEA did not reduce the cell death induced by HrpNea alone (). This result indicates that HrpNea induced KORC activationCitation12,Citation14 is not involved in cell death, mainly due to the decrease of anion current as previously proposed,Citation12 whereas it is involved in cell death triggered by simultaneous addition of HrpNea and HrpWea cell death. This comforts the hypothesis of a second way for harpins to induce cell death through an AVD type process ( and D).
Further studies are needed to improve the model presented in concerning the role of ion channels and their place in transduction mechanisms leading to harpin-induced cell death. Studies of particular interest would be the timing and quantification of the different harpins secreted during interaction.
Figures and Tables
Figure 1 Schema presenting the putative involvements of anion and K+ outward rectifying channels in the control of cell death triggered in non-host Arabidopsis cells by the two harpins of Erwinia amylovora, HrpN and HrpW. (A) Effect of 200 nM HrpN alone or mixed with 0.2 nM HrpW (B). (C) Effect of 200 nM HrpW alone or mixed with 200 nM HrpN (D).
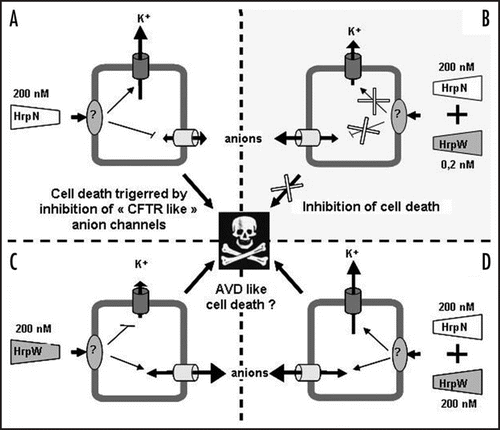
Figure 2 Simultaneous addition of 200 nM HrpWea and HrpNea triggers strong activation of anion channels and K+ outward rectifying channels (KORC) in suspension cell. (A) Whole cell current recordings before and after treatment with 200 nM HrpWea and HrpNea. Protocol was as indicated, Vh, holding potential; Vm, membrane potential. (B) Mean amplitude of steady state time dependent KORC at +80 mV. (C) Mean amplitudes of steady state anion currents at −200 mV. The control corresponds to the addition of an equivalent volume of the buffer used for harpin preparation. Values are given as a percentage with respect to current values before treatment. All values are mean of at least five independent experiments. Error bars correspond to standard errors.
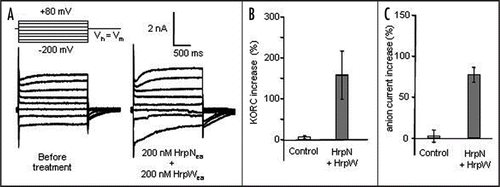
Figure 3 Effect of 5 mM TEA, a K+ channel inhibitor, on cell death triggered by 200 nM HrpNea or 200 nM HrpNea and HrpWea. Cell death was expressed as a percentage with respect to cell death level of nontreated cells. Data are means of 4 independent experiments and error bars correspond to standard errors.
Addendum to:
References
- He SY, Nomura K, Whittam TS. Type III protein secretion mechanism in mammalian and plant pathogens. Biochim Biophys Acta 2004; 1694:181 - 206
- Reboutier D, Frankart C, Briand J, Biligui B, Rona JP, Haapalainen M, Barny MA, Bouteau F. Antagonistic action of harpin proteins: HrpWea from Erwinia amylovora suppresses HrpNea induced cell death in Arabidopsis thaliana. J Cell Sci 2007; 120:3271 - 3278
- Viboud GI, Bliska JB. Yersinia outer proteins: Role in modulation of host cell signaling responses and pathogenesis. Annu Rev Microbiol 2005; 59:69 - 89
- Holeva MC, Bell KS, Hyman LJ, Avrova AO, Whisson SC, Birch PR, Toth IK. Use of a pooled transposon mutation grid to demonstrate roles in disease development for Erwinia carotovora subsp. atroseptica putative type III secreted effector (DspE/A) and helper (HrpN) proteins. Mol Plant Microbe Interact 2004; 17:943 - 950
- Reboutier D, Frankart C, Briand J, Biligui B, Laroche S, Rona JP, Barny MA, Bouteau F. The HrpNea harpin from Erwinia amylovora triggers differential desponses on the nonhost Arabidopsis thaliana cells and on the host apple cells. Mol Plant Microbe Interact 2007; 20:94 - 100
- Li CM, Haapalainen M, Lee J, Nurnberger T, Romantschuk M, Taira S. Harpin of Pseudomonas syringae pv. phaseolicola harbors a protein binding site. Mol Plant Microbe Interact 2005; 18:60 - 66
- Gaudriault S, Brisset MN, Barny MA. HrpW of Erwinia amylovora, a new Hrp-secreted protein. FEBS Lett 1998; 428:224 - 228
- Kim JF, Beer SV. HrpW of Erwinia amylovora, a new harpin that contains a domain homologous to pectate lyases of a distinct class. J Bacteriol 1998; 180:5203 - 5210
- Gauthier A, Lamotte O, Reboutier D, Bouteau F, Pugin A, Wendehenne D. Cryptogein-induced anion effluxes: Electrophysiological properties and analysis of the mechanisms through which they contribute to the elicitor-triggered cell death. Plant Signal Behav 2007; 2:89 - 98
- Blein JP, Milat ML, Ricci P. Responses of cultured tobacco cells to cryptogein, a proteinaceous elicitor from Phytophtora cryptogea: Possible plasmalemma involvement. Plant Physiol 1991; 95:486 - 491
- Okada Y, Maeno E. Apoptosis, cell volume regulation and volume-regulatory chloride channels. Comp Biochem Physiol A Mol Integr Physiol 2001; 130:377 - 383
- Reboutier D, Frankart C, Vedel R, Brault M, Duggleby RG, Rona JP, Barny MA, Bouteau F. A CFTR chloride channel activator prevents HrpN(ea)-induced cell death in Arabidopsis thaliana suspension cells. Plant Physiol Biochem 2005; 43:567 - 572
- Wendehenne D, Lamotte O, Frachisse JM, Barbier-Brygoo H, Pugin A. Nitrate efflux is an essential component of the cryptogein signaling pathway leading to defense responses and hypersensitive cell death in tobacco. Plant Cell 2002; 14:1937 - 1951
- El-Maarouf H, Barny MA, Rona JP, Bouteau F. Harpin, a hypersensitive response elicitor from Erwinia amylovora, regulates ion channel activities in Arabidopsis thaliana suspension cells. FEBS Lett 2001; 497:82 - 84