Abstract
The orientation of plant root growth is modulated by developmental as well as environmental cues. Among the environmental factors, gravity has been extensively studied because of its overpowering effects in modulating root growth direction. However, our knowledge of the effects of other abiotic signals that influence root growth direction is largely unknown. Recently, we have shown that high salinity can modify root growth direction by inducing rapid amyloplast degradation in root columella cells of Arabidopsis thaliana. By exploiting salt overly sensitive (sos) mutants and PIN2 expression analyses, we have shown that the altered root growth direction in response to salt is mediated by ion disequilibrium and is correlated with PIN2 mRNA abundance and expression and localization of the protein. Our study demonstrates that the SOS pathway may mediate this process. Here we discuss our data from broader perspectives. We propose that salt-induced modification of root growth direction is a salt-avoidance behavior, which is an active adaptive mechanism for plants grown under saline conditions. Furthermore, high salinity also stimulates alteration of gravitropic growth of shoots. These findings illustrate that plants have a fine and sophisticated sensory and communication system that enable plants to dynamically and efficiently cope with rapidly changing environment.
Owing to their sessile nature, plant roots are constantly bombarded with various environmental stimuli from the soil, such as gravity, physical obstacles and imbalanced distribution of water and/or nutrients and high salinity. Where to grow is an important developmental decision in the life cycle of a plant that is crucial for its adaptation and the subsequent reproductive success. The proper orientation of root growth is shaped by both the developmental inputs and external signals.Citation1,Citation2 The overwhelming environmental factor that modulates root growth direction is gravity, and plant primary roots grow downward toward the gravity vector. This directed growth of root in response to gravity is named as tropic growth to gravity or gravitropism. Studies of gravity perception and signaling pathway of the root cap at the primary root of Arabidopsis strongly support the starch statolith hypothesis.Citation3 In this hypothesis, the columella cells in the root cap, which contain sedimentable amyloplasts, are the gravity-perceptive site in roots. The inner columella cells of the second tier have been proposed as making the greatest contribution to root gravitropism.Citation4 Upon gravity stimulation, cytosolic ions such as Ca2+ and rapid cytoplasmic alkalization may be involved in gravity signal transduction.Citation5–Citation7 Asymmetric distribution of auxin in roots caused by basipetal transport mainly through the auxin efflux carrier PIN-FORMED2 (PIN2), which is distributed asymmetrically within the cells, results in gravitropic root response of the root elongation zone.Citation8,Citation9
In contrast to our understanding of gravitropism of root, our knowledge of tropistic responses of root to other major environmental stimuli, such as water availability, imbalanced nutrient distribution and high salinity, and the interplay between these stimuli in determining the directional growth of root remains enigmatic. Recent studies have confirmed the existence of hydrotropism and the molecular genetic basis of the tropistic growth of root to water in determining the final direction of root growth starts to be deciphered.Citation10–Citation12 Hydrotropic growth of roots is an important trait for plants to actively find water and to optimize their fitness under drought condition. Salinity is another major constraint to root system development, and limits the productivity of agricultural crops and the distribution of plant species.Citation13–Citation15 It is known that salt stress-induced disturbed balance of ions is the primary cause for inhibition of plant growth and subsequent yield reduction. How does root minimize entrance of harmful ions and subsequently avoid salt injury? Does plant have capacity to sense salt signal, and prevent potentially harmful ions reaching root and shoot?
Previous studies have shown that plant use different strategies to avoid salt injury at various levels. After Na+ enters the root cells, the Casparian strip can restrict the movements of the harmful ion into the xylem.Citation16 Root cells also avoid salt injury by extruding Na+ actively back to the outside solution. This energy-dependent ion efflux from cytosol across the plasma membrane is mediated by SOS1 gene, a Na+-H+ antiporter, which is regulated by at least other genes, SOS3 (calcium binding protein) and SOS2 (serine/threonine kinase). This is the well characterized SOS (Salt Overly Sensitive) signaling pathway.Citation17,Citation18 Another way for plant root cells to avoid ion injury is to accumulate Na+ into vacuole. Vacuolar compartmentation of Na+ is also in part regulated by Na+-H+ antiporters, such as AtNHX1.Citation19 These findings reveal mechanisms of how plants avoid Na+ injury after passive entrance of sodium ions into root cells. We questioned whether a plant is capable of actively preventing the harmful ions from reaching root cells or escape from high salinity in the environment, and how plant roots respond to changing salt conditions, because salt distribution is unbalanced under natural saline conditions, especially after rain and irrigation. With a new assay that allows us to specifically address how plant roots respond to changing salt levels, we discovered an alternative adaptive mechanism for plant root to avoid salt injury.Citation20
We set up a two-layer medium assay in which a sodium ion gradient would be generated. A normal nutrient agar medium is at the top of the growth bottle and an agar with salt-stressed medium is in the bottom of the bottle. This simple assay allows us to monitor root growth and orientation. The roots of the wild type seedlings penetrated the interface of the layers and grew straight downwards exhibiting gravitropism, when both layers were MS media. In contrast, when the bottom medium contained NaCl, roots of seedlings grew downward first, and then curved and grew upward toward the lower levels of salt. Roots started to bend upward at an early stage even before contacting high-salt medium (250 mM NaCl) on the bottom. The results indicate that roots can sense ion gradients in the growing environment and transduce the signal, combine with internal signals to make decisions that enable roots to stay away from high salt.Citation21,Citation22 Here, we would like to propose this salt-induced tropic growth as a salt-avoidance tropism, which is an important adaptive behavior for plant roots to avoid salt injury and direct them toward their goal of optimal fitness.Citation23 Because salt stress inhibits root elongation, we tested impact of salt-induced negative gravitropism on the root growth. The results showed that inhibitory effect of salt on root growth was largely alleviated with this tropic curve (), further verifying our hypothesis that the salt-induced developmental plasticity is a salt-avoidance behavior ().
Another important point that we would like to bring out based on our observation in this work is that salinity also stimulated shoot positive gravitropism or negative phototropism. The observation implicates long-distance communication from root to shoot during plant salt response in the stressed plants. The exact biological function of shoot tropic growth, the signals in this long-distance communication, and underlying molecular mechanism still remains unknown.
In conclusion, our study has revealed a novel complex adaptive mechanism that provides plants a capacity for avoiding injury from salt. The hypothesis we have proposed here should provide novel insights into plant stress avoidance. Further analysis using a combinatorial approach, mutant analysis and genomics, is required to decipher the molecular network underlying this salt-avoidance behavior.
Figures and Tables
Figure 1 Effects of salt on root elongation of Arabidopsis thaliana seedlings from different salt treatments. The inhibitory effect of salt stress on root growth was greatly alleviated in the wild type (Col-0) when root growth of the seedlings was analyzed using a two-layer medium assay (black bars). The MS nutrient medium is on the top, and NaCl concentrations in the media on the bottom are 0, 150 and 250 mM. More severe inhibition of root growth of the seedlings by various levels of NaCl in a root bending assay (white bars) was observed. Data represents means of measurements from >40 individuals from three independent experiments. Bars represent standard error.
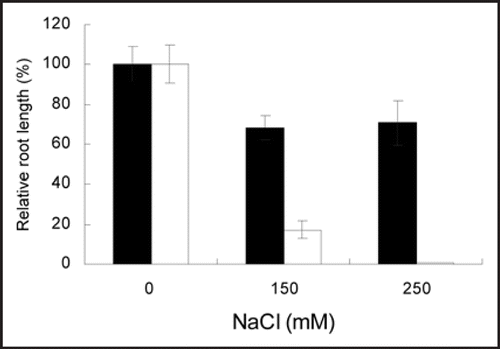
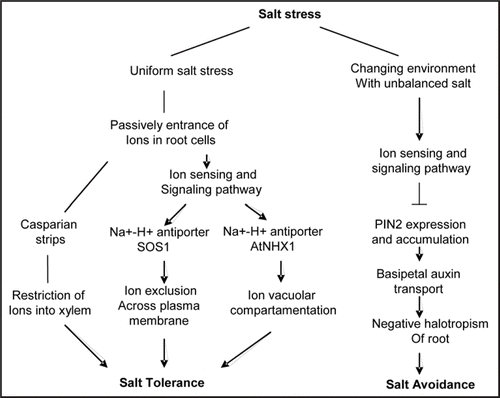
Figure 2 An illustrative model of the sensing and response by the plant root when grown under different saline conditions. This model proposes two major mechanisms of salt responses by plants, where salt tolerance is the ability to function while stressed; Salt avoidance is the capacity to stay away from salt stress when growing in changing saline conditions.
Addendum to:
References
- Kiss JZ. Where's the water? Hydrotropism in plants. Proc Natl Acad Sci USA 2007; 104:4247 - 4248
- Kiss JZ, Correll MJ, Mullen JL, Hangarter RP, Edelmann RE. Root phototropism: how light and gravity interact in shaping plant form. Gravit Space Biol Bull 2003; 16:55 - 60
- Morita MT, Tasaka M. Gravity sensing and signaling. Curr Opin Plant Biol 2004; 7:712 - 718
- Kiss JZ, Hertel R, Sack FD. Amyloplasts are necessary for full gravitropic sensitivity in roots of Arabidopsis thaliana. Planta 1989; 177:198 - 206
- Johannes E, Collings DA, Rink JC, Allen NS. Cytoplasmic pH dynamics in maize pulvinal cells induced by gravity vector changes. Plant Physiol 2001; 127:119 - 130
- Fasano JM, Swanson SJ, Blancaflor EB, Dowd PE, Kao TH, Gilroy S. Changes in root cap pH are required for the gravity response of the Arabidopsis root. The Plant Cell 2001; 13:907 - 921
- Legue V, Blancaflor E, Wymer C, Perbal G, Fantin D, Gilroy S. Cytoplasmic free Ca2+ in Arabidopsis roots changes in response to touch but not gravity. Plant Physiol 1997; 114:789 - 800
- Abas L, Benjamins R, Malenica N, Paciorek T, Wisniewska J, Moulinier-Anzola JC, Sieberer T, Friml J, Luschnig C. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat Cell Biol 2006; 8:249 - 256
- Ottenschlager I, Wolff P, Wolverton C, Bhalerao RP, Sandberg G, Ishikawa H, Evans M, Palme K. Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc Natl Acad Sci USA 2003; 100:2987 - 2991
- Eapen D, Barroso ML, Campos ME, Ponce G, Campos ME, Cassab GI. Hydrotropsm: root growth responses to water. Trends Plant Sci 2005; 10:44 - 50
- Kobayashi A, Takahashi A, Kakimoto Y, Miyazawa Y, Fujii N, Higashitani A, Takahashi H. A gene essential for hydrotropism in roots. Proc Natl Acade Sci USA 2007; 104:4724 - 4729
- Takahashi N, Yamazaki Y, Kobayashi A, Higashitani A, Takahashi H. Hydrotropism interacts with gravitropism by degrading amyloplasts in seedling roots of Arabidopsis and radish. Plant Physiol 2003; 132:805 - 810
- Flowers TJ, Yeo AR. Breeding for salinity resistance in crop plants: Where next?. Aust J Pl Physiol 1995; 22:875 - 884
- Zhu JK. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 2002; 53:247 - 273
- Zhu JK. Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 2003; 6:441 - 445
- Enstone DE, Peterson CA, Ma F. Root endodermis and exodermis: Structure, function, and responses to the environment. J Plant Growth Regul 2003; 21:335 - 351
- Zhu JK, Liu J, Xiong L. Genetic analysis of salt tolerance in Arabidopsis: evidence for a role of potassium nutrition. Plant Cell 1998; 10:1181 - 1191
- Shi H, Ishitani M, Kim C. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc Natl Acad Sci USA 97:6891 - 6901
- Apse MP, Aharon GS, Snedden WA, Blumwald E. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 1999; 285:1256 - 1258
- Sun F, Zhang W, Hu H, Li B, Wang Y, Zhao Y, Li K, Liu M, Li X. Salt modulates gravity signaling pathway to regulate growth direction of primary roots in Arabidopsis thaliana. Plant Physiol 2008; 146:178 - 188
- Lynch J. Root architecture and plant productivity. Plant Physiol 1995; 109:7 - 13
- Malamy JE. Intrinsic and environmental response pathways that regulate system architecture. Plant Cell Environ 2005; 28:67 - 77
- Trewavas A. Green plants as intelligent organisms. Trends Plant Sci 2005; 10:1360 - 1385