Abstract
Tonoplast-localized proton-coupled Ca2+ transporters encoded by cation/H+ exchanger (CAX) genes play a critical role in sequestering Ca2+ into the vacuole. These transporters may function in coordination with Ca2+ release channels, to shape stimulus-induced cytosolic Ca2+ elevations. Recent analysis of Arabidopsis CAX knockout mutants, particularly cax1 and cax3, identified a variety of phenotypes including sensitivity to abiotic stresses, which indicated that these transporters might play a role in mediating the plant’s stress response. A common feature of these mutants was the perturbation of H+-ATPase activity at both the tonoplast and the plasma membrane, suggesting a tight interplay between the Ca2+/H+ exchangers and H+ pumps. We speculate that indirect regulation of proton flux by the exchangers may be as important as the direct regulation of Ca2+ flux. These results suggest cautious interpretation of mutant Ca2+/H+ exchanger phenotypes that may be due to either perturbed Ca2+ or H+ transport.
Ca2+ plays a fundamental role in the plant cell, functioning as a highly versatile second messenger controlling a multitude of cellular reactions and adaptive responses.Citation1,Citation2 Ca2+ dynamics are maintained by precise interplay among transporters involved in its release from or uptake into Ca2+ stores. The vacuole, as the largest internal Ca2+ pool, is assumed to play a major role in Ca2+ signalling, and has been shown to be the source of Ca2+ release following various abiotic stresses such as cold and osmotic stress.Citation3,Citation4 Rapid, stimulus-induced release of Ca2+ from the vacuole is attributable to selectively permeable Ca2+ channels, however, the identity of candidate genes encoding this mechanism remains contested.Citation5,Citation6 Better understood, are the two major vacuolar uptake mechanisms; P-type Ca2+ pumps, including ACA4 and ACA11, which mediate high-affinity Ca2+ uptake, and a family of cation/H+ exchangers (CAX), responsible for lower-affinity but high-capacity Ca2+ uptake.Citation7,Citation8 While Ca2+ pumps rely directly on the hydrolysis of ATP to drive Ca2+ uptake, Ca2+/H+ exchangers are energized indirectly by the pH gradient generated by electrogenic H+ pumps located on the tonoplast, including the vacuolar-type H+-ATPase (V-ATPase).Citation9
With the aim of further understanding the role of specific CAX isoforms in Arabidopsis, we and others have recently characterized CAX mutants and overexpression lines and observed a variety of phenotypes, including altered response to abiotic stresses.Citation10–Citation14 While some phenotypes are identical among different CAX mutants, others are specific to individual lines.Citation14 Moreover, these analyses have highlighted the interplay of these transporters with H+ pumps at both the tonoplast and the plasma membrane. Overexpression of CAX1 in Arabidopsis results in increased activity of the V-ATPase, whereas mutations in CAX1 cause a concomitant decrease in measured V-ATPase activity ().Citation11 Similar reductions in V-ATPase activity are also observed in cax2 and cax3 mutant plants but to a lesser extent,Citation12,Citation13 and a significant reduction is observed in a cax1 cax3 double knockout line.Citation13 At the plasma membrane, P-type H+-ATPase (P-ATPase) activity is increased in cax1 but decreased in cax3 ().Citation14 Indeed cax3 lines appeared more sensitive to changes in the pH of the growth media.Citation14 This implies that unlike cax1, cax3 is less efficient at cytoplasmic pH adjustment. Another intriguing observation is that activity of the H+-pyrophosphatase (H+-PPase) at the tonoplast is largely unaltered following CAX gene deletion. While overexpression of the Arabidopsis H+-PPase AVP1 leads to increased Ca2+/H+ exchange activity,Citation15,Citation16 there is little alteration in H+-PPase activity following perturbed expression of CAX1 or CAX2.Citation11,Citation12 Thus, this feedback interplay appears to exist only between exchangers and H+-ATPases.
The V-ATPase is important not only for maintenance of a pH gradient across the tonoplast, but also in maintenance of Golgi structure, endocytosis and secretory trafficking.Citation17,Citation18 The V-ATPase is localised at the Golgi, endoplasmic reticulum and endosomes, in addition to the tonoplast.Citation9 The det3 mutant, with a mutation in subunit C (VHA-C), has a 40–60% reduction in V-ATPase activity, but numerous severe developmental phenotypes.Citation19 In contrast, the cax1 and cax1 cax3 mutants have a reduction in V-ATPase activity equivalent to det3 (), but the morphological phenotypes are not as pronounced.Citation13 It is therefore likely that reduction of tonoplast Ca2+/H+ exchange primarily affects tonoplast V-ATPase activity, while V-ATPase activity in the secretory pathway is unperturbed. The V-ATPase is a multi-subunit protein and some of these subunit gene products appear to be either tonoplast-specific or tonoplast-enriched. Mutations in tonoplast subunits may cause defective V-ATPase activity only at the tonoplast.Citation9 It will be of interest to see whether such tonoplast-specific V-ATPase mutants phenocopy the cax mutants, and possess perturbed Ca2+/H+ exchange activity and altered abiotic stress responses.
CAX-mediated transport may alter both cytoplasmic and lumenal pH, as well as intracellular Ca2+ gradients. In the case of the V-ATPase, evidence is emerging for a role not only in the generation of a pH gradient across membranes, but also in the direct sensing of pH within the compartment,Citation20,Citation21 creating a feedback mechanism which regulates pump activity. Thus, in cax1 lines, abnormal acidification of the lumen is detected by the V-ATPase resulting in a dampening of its activity. This would conserve ATP, which we postulate could be utilized to drive the tonoplast Ca2+ pump which itself is upregulated in cax1 as a compensatory mechanism to correct perturbations in the Ca2+ gradient.Citation11 In the case of cax1, this in turn may signal the P-ATPase to remove surplus H+ from the cytoplasm, triggering its upregulation (). However, not all CAX mutants show this complex H+ feedback mechanism.
Co-ordinate downregulation of the V-ATPase in the cax1 mutant lines may also be a result of activity of the SOS2 kinase. This Ser/Thr kinase, which specifically interacts with the N-terminus of CAX1 resulting in Ca2+/H+ exchange activation,Citation22 upregulates V-ATPase activity through interactions with the VHA-B regulatory subunit.Citation23 Loss of CAX1 may be signalling to the V-ATPase through changes in SOS2 activity resulting in a compensatory downregulation of the pump. It is tempting to speculate that SOS2 may signal the alteration in P-ATPase activity, as it is known to regulate other plasma membrane proteins, notably the Na+/H+ exchanger SOS1.Citation24 It will be interesting to determine if SOS2 and the P-ATPase interact directly. It is notable, however, that SOS2 does not appear to interact with CAX3,Citation22 while P-ATPase activity is reduced in cax3 plants.Citation14
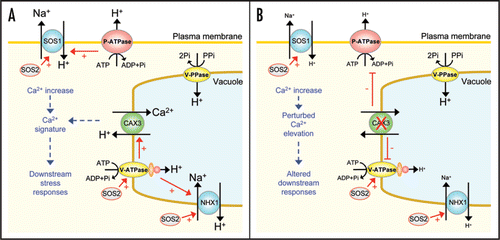
Our recent results indicate there are at least two modes by which Ca2+/H+ exchangers can mediate adaptive responses to stress: direct manipulation of cytosolic Ca2+ and indirect feedback of H+ flux (). For example, salt stress responses are likely controlled via the generation of a specific cytosolic Ca2+ signature, which mediates a downstream signalling pathway. CAX3 appears to be the principle isoform providing tonoplast Ca2+/H+ exchange in response to salt stress.Citation14 Disruption of CAX3-mediated tonoplast Ca2+ transport and the alteration of cytosolic Ca2+ dynamics may therefore alter the plant's normal response to salt stress (). Maintenance of H+ gradients at both the vacuole and plasma membrane are also critical for salt tolerance, such that salt treatment upregulates V-ATPase and P-ATPase activity.Citation25 This energizes Na+ efflux from the cytosol mediated by Na+/H+ exchangers at the plasma membrane and the tonoplast.Citation24,Citation26 Therefore downregulation of H+ pumps at both membranes in the cax3 mutant is likely to perturb the ability of the cell to remove Na+ (). Further analysis of cax mutants, P-ATPase mutants, and tonoplast-specific V-ATPase mutants will be required to determine whether many of the phenotypes resulting from lack of Ca2+/H+ exchange activity are due to altered Ca2+ transport or H+ transport.
The phenomenon observed between tonoplast Ca2+/H+ exchangers and H+ pumps at both the tonoplast and plasma membranes, suggesting a co-ordinate regulation between several transporters, is not solely restricted to this family of transporters. It is a common observation emerging from recent research on the manipulation of tonoplast transporters. Several labs have reported unpredictable phenotypes associated with ectopic expression of tonoplast proteins.Citation26–Citation28 Until we understand the significance of these types of unexpected interactions, it is naïve to believe that engineering plants will provide predictable results.
Figures and Tables
Figure 1 Tonoplast H+-ATPase (V-ATPase) activity and plasma membrane H+-ATPase (P-ATPase) activity in wild type Arabidopsis (ecotype Col-0) and Arabidopsis lines with manipulated tonoplast Ca2+/H+ exchange activity. 35S::CAX1 and 35S::CAX2 denote lines that overexpress a constitutively active N-terminally truncated CAX1 or CAX2 construct driven by the CaMV 35S promoter in the cax1-1 or cax2-1 mutant background, respectively. V-ATPase H+-transport activity was measured by the ATP-dependent quenching of quinacrine fluorescence, and rates of bafilomycin-sensitive, vanadate-resistant hydrolytic activity of the V-ATPase were determined in isolated tonoplast membranes, as described in refs. Citation11 and Citation13. Rates of vanadate-sensitive, bafilomycin- and azide-resistant hydrolytic activity of the P-ATPase were determined in isolated plasma membranes, as described in ref. Citation14. Results are shown as % of wild type (Col-0) ATPase activity and are means ± SE of 3–4 independent experiments. Data taken and modified from refs. Citation11–Citation14.
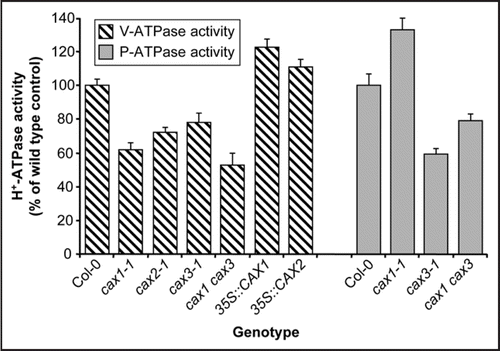
Figure 2 Model of tonoplast Ca2+/H+ exchanger interaction with H+ pumps in response to salt stress. (A) In response to NaCl treatment, an elevation in cytosolic Ca2+ will occur, possibly due to vacuolar Ca2+ release.Citation3 Increased CAX3-mediated Ca2+/H+ exchange activityCitation14 will sequester excess Ca2+ into the vacuole. CAX3 may be involved in the generation of a specific Ca2+ signature that is recognised by the cell to mediate downstream stress responses. In addition, salt stress will lead to upregulation of H+ pumps at both the plasma membrane and the tonoplast (P-ATPase and V-ATPase)Citation25 which will in turn energize Na+/H+ exchange activity encoded by SOS1 and NHX1, promoting Na+ efflux from the cell. Increased V-ATPase activity may also upregulate Ca2+/H+ exchange. Activity of SOS1 requires activation by the kinase SOS2Citation24 which may also regulate tonoplast Na+/H+ exchange and V-ATPase activity.Citation23,Citation24 (B) In a cax3 knockout mutant experiencing salt stress, the cytosolic Ca2+ elevation may be sustained due to reduced vacuolar Ca2+ sequestration and normal salinity-induced Ca2+ signalling pathways may be perturbed. Lack of CAX3 downregulates both P-ATPase and V-ATPase activityCitation14 thereby reducing energization of the plasma membrane and tonoplast Na+/H+ exchangers and reducing Na+ efflux from the cell. Some energization of H+-coupled processes at the vacuole may be maintained by residual H+-pyrophosphatase (V-PPase) activity.
Acknowledgements
We acknowledge the financial support of a CONACyT grant 49735 to B.J.B., a National Science Foundation grant #90344350 and USDA-CSREES #2005-34402-17121, Designing Foods for Health to K.D.H., and a Biotechnology and Biological Sciences Research Council David Phillips Research Fellowship grant BB/B5021521 to J.K.P. We thank James Connorton for comments on the manuscript.
Addendum to:
References
- Sanders D, Pelloux J, Brownlee C, Harper JF. Calcium at the crossroads of signaling. Plant Cell 2002; 14:401 - 417
- Hetherington AM, Brownlee C. The generation of Ca2+ signals in plants. Annu Rev Plant Biol 2004; 55:401 - 427
- Knight H, Trewavas AJ, Knight MR. Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J 1997; 12:1067 - 1078
- Knight H, Knight MR. Imaging spatial and cellular characteristics of low temperature calcium signature after cold acclimation in Arabidopsis. J Exp Bot 2000; 51:1679 - 1686
- Ranf S, Wünnenberg P, Lee J, Becker D, Dunkel M, Hedrich R, Scheel D, Dietrich P. Loss of the vacuolar cation channel, AtTPC1, does not impair Ca2+ signals induced by abiotic and biotic stresses. Plant J 2008; 53:287 - 299
- Peiter E, Maathuis FJ, Mills LN, Knight H, Pelloux J, Hetherington AM, Sanders D. The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature 2005; 434:404 - 408
- Pittman JK, Hirschi KD. Don't shoot the (second) messenger: endomembrane transporters and binding proteins modulate cytosolic Ca2+ levels. Curr Opin Plant Biol 2003; 6:257 - 262
- Shigaki T, Hirschi KD. Diverse functions and molecular properties emerging for CAX cation/H+ exchangers in plants. Plant Biol 2006; 8:419 - 429
- Schumacher K. Endomembrane proton pumps: connecting membrane and vesicle transport. Curr Opin Plant Biol 2006; 9:595 - 600
- Catala R, Santos E, Alonso JM, Ecker JR, Martinez-Zapater JM, Salinas J. Mutations in the Ca2+/H+ transporter CAX1 increase CBF/DREB1 expression and the cold-acclimation response in Arabidopsis. Plant Cell 2003; 15:2940 - 2951
- Cheng NH, Pittman JK, Barkla BJ, Shigaki T, Hirschi KD. The Arabidopsis cax1 mutant exhibits impaired ion homeostasis, development and hormonal responses, and reveals interplay among vacuolar transporters. Plant Cell 2003; 15:347 - 364
- Pittman JK, Shigaki T, Marshall JL, Morris JL, Cheng N-H, Hirschi KD. Functional and regulatory analysis of the Arabidopsis thaliana CAX2 cation transporter. Plant Mol Biol 2004; 56:959 - 971
- Cheng NH, Pittman JK, Shigaki T, Lachmansingh J, LeClere S, Lahner B, Salt DE, Hirschi KD. Functional association of Arabidopsis CAX1 and CAX3 is required for normal growth and ion homeostasis. Plant Physiol 2005; 138:2048 - 2060
- Zhao J, Barkla BJ, Marshall J, Pittman JK, Hirschi KD. The Arabidopsis cax3 mutants display altered salt tolerance, pH sensitivity and reduced plasma membrane H+-ATPase activity. Planta 2008; 227:659 - 669
- Gaxiola RA, Li J, Undurraga S, Dang LM, Allen GJ, Alper SL, Fink GR. Drought- and salt-tolerant plants result from overexpression of the AVP1 H+-pump. Proc Natl Acad Sci USA 2001; 98:11444 - 11449
- Park S, Li J, Pittman JK, Berkowitz GA, Yang H, Undurraga S, Morris J, Hirschi KD, Gaxiola RA. Upregulation of a H+-pyrophosphatase (H+-PPase) as a strategy to engineer drought-resistant crop plants. Proc Natl Acad Sci USA 2005; 102:18830 - 18835
- Strompen G, Dettmer J, Stierhof YD, Schumacher K, Jurgens G, Mayer U. Arabidopsis vacuolar H-ATPase subunit E isoform 1 is required for Golgi organization and vacuole function in embryogenesis. Plant J 2005; 41:125 - 132
- Dettmer J, Hong-Hermesdorf A, Stierhof YD, Schumacher K. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 2006; 18:715 - 730
- Schumacher K, Vafeados D, McCarthy M, Sze H, Wilkins T, Chory J. The Arabidopsis det3 mutant reveals a central role for the vacuolar H+-ATPase in plant growth and development. Genes Dev 1999; 13:3259 - 3270
- Marshansky V. The V-ATPase a2-subunit as a putative endosomal pH-sensor. Biochem Soc Trans 2007; 35:1092 - 1099
- Hurtado Lorenzo A, Skinner M, El Annan J, Futai M, Sun Wada GH, Bourgoin S, Casanova J, Wildeman A, Bechoua S, Ausiello DA, Brown D, Marshansky V. V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat Cell Biol 2006; 8:124 - 139
- Cheng NH, Pittman JK, Zhu JK, Hirschi KD. The protein kinase SOS2 activates the Arabidopsis H+/Ca2+ antiporter CAX1 to integrate calcium transport and salt tolerance. J Biol Chem 2004; 279:2922 - 2926
- Batelli G, Verslues PE, Agius F, Qiu Q, Fujii H, Pan S, Schumaker KS, Grillo S, Zhu JK. SOS2 promotes salt tolerance in part by interacting with the vacuolar H+-ATPase and upregulating its transport activity. Mol Cell Biol 2007; 27:7781 - 7790
- Zhu JK. Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 2003; 6:441 - 445
- Janicka Russak M, Klobus G. Modification of plasma membrane and vacuolar H+-ATPases in response to NaCl and ABA. J Plant Physiol 2007; 164:295 - 302
- Zhang HX, Blumwald E. Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat Biotech 2001; 19:765 - 768
- Li J, Yang H, Peer WA, Richter G, Blakeslee J, Bandyopadhyay A, Titapiwantakun B, Undurraga S, Khodakovskaya M, Richards EL, Krizek B, Murphy AS, Gilroy S, Gaxiola R. Arabidopsis H+-PPase AVP1 regulates auxin-mediated organ development. Science 2005; 310:121 - 125
- Shaul O, Hilgemann DW, de-Almeida-Engler J, Van Montagu M, Inze D, Galili G. Cloning and characterization of a novel Mg2+/H+ exchanger. EMBO J 1999; 8:3973 - 3980