Abstract
Recently we reported the identification of a novel B-box transcription factor SALT TOLERANCE HOMOLOG 2 (STH2) that interacts genetically with two key regulators of the light-signaling pathway, HY5 and COP1. We also provided phenotypic and genetic characterization of the sth2 mutant suggesting that STH2 plays a positive role in regulating photomorphogenesis both independently or together with HY5. Functional assays in protoplasts revealed that STH2 could act as a transcriptional activator. To our knowledge this is the first report of a B-box domain containing protein playing a direct role in activating transcription in plants. Here we discuss the possible position of STH2 in the transcriptional network and comment on the role of the B-box domain in plants.
Biology of Interactions between STH2, HY5 and COP1
Light induces massive reprogramming of the plant transcriptome, and many of the early light-responsive genes are transcription factors. LONG HYPOCOTYL 5 (HY5) is a high hierarchical regulator of the transcriptional cascades for photomorphogenesis and acts downstream to the photoreceptors.Citation2 It is constitutively nuclear, binds to the promoters of light inducible genes and regulates their expression during photomorphogenesis. The activity of HY5 is in turn controlled by a key negative regulator, CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1) that degrades HY5 in the dark.Citation3 We recently identified STH2, a B-box containing protein that interacts genetically with both HY5 and COP1Citation1. Phenotypes like hyposensitivity to blue, red and far-red light, enhanced number of lateral roots and reduced anthocyanin accumulation indicate that STH2 plays a positive role in photomorphogenesis. We found that STH2 could activate transcription and showed that the B-boxes in STH2 and a functional G-box element in the promoter are required for the activity. The fact that HY5 has been found to constitutively bind to the promoters of a set of genes related to photosynthesis and circadian regulation, such as RbcS1A, CHS, CCA1 and TOC1 irrespective of the light-dark transition or the daily rhythm suggests that HY5 binding is not sufficient for transcriptional activation and might require some additional cofactors for regulation.Citation2 Our result suggests that STH2 and possibly other B-box containing proteins could be the additional factors HY5 needs for transcriptional regulation. Furthermore STH2 might also act as a cofactor for other G-box binding proteins such as HYH regulating HY5 independent processes.Citation4,Citation5 Our data showing COP1-dependent localization of STH2 to speckles and genetic interactions between sth2 and cop1 both in the dark and in light suggests that the B-box protein STH2, the bZIP transcription factor HY5 and the RING finger and coiled-coil domain containing COP1 protein work in close proximity to regulate photomorphogenesis in Arabidopsis.
RBCC/TRIM Family Proteins
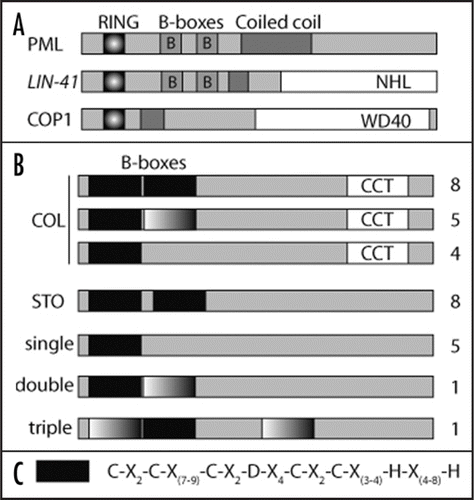
The B-box is a zinc-ligating domain consisting of conserved Cys and His residues.Citation6 In animals B-boxes are mostly found in conjugation with a RING finger domain (originally termed an A-box) and a coiled-coil domain forming RBCC or tripartite motif proteins. The RBCC family includes a large number of proteins involved in various cellular processes like apoptosis, cell cycle regulation and viral response.Citation7 Recently a number of TRIM/RBCC proteins () have been found to play a role in ubiquitination and the B-boxes proposed to participate in substrate recognition. Other functions of this domain involve localization into nuclear bodies as in the tumor suppressor protein PML (Promyelocytic Leukemia), transcriptional regulationCitation8 and protein-protein interaction. The B-box domain has been classified into two subgroups, B-box1 and B-box2, both of which have seven or eight conserved Cys and His residues to coordinate two zinc atoms in a ring-like fold. B-boxes are usually found as single domains or as tandem repeats. While B-box2 is present in all RBCC proteins, B-box1 is absent in proteins containing only one B-box and is present N-terminal to B-box2 in proteins containing two tandemly repeated B-boxes.
B-box Proteins in Plants
Although RBCC proteins are absent in Arabidopsis, there are 32 proteins with N-terminal B-boxes (). In contrast to animals all Arabidopsis B-box containing proteins have at least one B-box with an Asp as the fourth zinc-coordinating residue. The consensus sequence of this B-box is shown in . A large subgroup (the 17 COL proteins) of this family contains an additional CCT domain in the C-terminal part of the protein.Citation9 STO, STH1 and STH2 together with five other proteins form a small subgroup, with two tandem repeated B-boxes with high homology. A third subgroup consists of five proteins, which contains only one B-box. Some other variants of B-boxes in Arabidopsis contain a Glu or His residue instead of the Asp as the fourth zinc-coordinating residue ().
Role of B-Boxes
Although the B-box has been proposed to be a protein interaction domain, its molecular function in plants is not well understood. Recently it was reported that the CCT domain of the B-box containing protein CONSTANS (CO) was involved in the formation of a heterotrimeric DNA binding complex suggesting that in plants B-box proteins have evolved to attain different functional roles.Citation10 Our yeast data showed that a structurally intact B-box domain was important for interaction with HY5, providing evidence for the role of B-box domain in protein-protein interaction. Furthermore, transient transfection assay in protoplasts indicated that a functional B-box domain is required to activate transcription, thus providing a novel molecular function for this domain. We propose a model wherein STH2 acts as a transcriptional cofactor that interacts with HY5 through its B-boxes and is able to activate transcription through the G-box promoter element.
Conclusions and Outlook
The fact that COP1 could recruit STH2 to nuclear speckles and the genetic interaction between sth2 and cop1 is interesting. Previously we had reported interaction between COP1 and the B-box containing COL3, STH1 and STO proteins.Citation11,Citation12 Recent results suggest that coiled-coil domain containing SPA proteins are important for the stability of the B-box containing protein CO.Citation13 All these interactions between B-box containing proteins and the RING, coiled-coil domain containing COP1-SPA proteins suggest a mechanism of creating a functional equivalent of RBCC protein in an organism that lacks such proteins. Whether these interacting B-box proteins together with COP1 play a role in ubiquitination and proteolysis or act as a substrate for COP1 mediated degradation is a question yet unanswered. However studies of these biochemical complexes might help unravel the functional intricacies of manifold cellular processes regulated by B-box containing proteins.
Figures and Tables
Figure 1 (A) Schematic representation of domains present in PML and LIN-41, two RBCC proteins found in animals and the Ring finger, coiled-coil domain containing COP1 protein. NHL and WD40 are protein interaction domains. (B) Classification and schematic representation of the 32 B-box containing proteins in Arabidopsis. Black boxes represent B-boxes present in all Arabidopsis proteins, whereas graded boxes indicate a variant of B-box containing Glu or His as the fourth zinc-coordinating residue. CCT represents the CO, CO-like, TOC1 domain. Numbers indicate members in each class. (C) Consensus sequence of the B-box found in all Arabidopsis proteins containing an Asp as the fourth zinc-coordinating residue.
Addendum to:
References
- Datta S, Hettiarachchi C, Johansson H, Holm M. SALT TOLERANCE HOMOLOG2, a B-box protein in Arabidopsis that activates transcription and positively regulates light-mediated development. Plant Cell 2007; 19:3242 - 3255
- Lee J, He K, Stolc V, Lee H, Figueroa P, Gao Y, Tongprasit W, Zhao H, Lee I, Deng XW. Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 2007; 19:731 - 749
- Ang LH, Chattopadhyay S, Wei N, Oyama T, Okada K, Batschauer A, Deng XW. Molecular interaction between COP1 and HY5 defines a regulatory switch for light control of Arabidopsis development. Mol Cell 1998; 1:213 - 222
- Sibout R, Sukumar P, Hettiarachchi C, Holm M, Muday GK, Hardtke CS. Opposite root growth phenotypes of hy5 versus hy5 hyh mutants correlate with increased constitutive auxin signaling. PLoS Genet 2006; 2:202
- Holm M, Ma LG, Qu LJ, Deng XW. Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes & Dev 2002; 16:1247 - 1259
- Torok M, Etkin LD. Two B or not two B? Overview of the rapidly expanding B-box family of proteins. Diff Res Biol Div 2001; 67:63 - 71
- Meroni G, Diez-Roux G. TRIM/RBCC, a novel class of ‘single protein RING finger’ E3 ubiquitin ligases. Bioessays 2005; 27:1147 - 1157
- Beenders B, Jones PL, Bellini M. The tripartite motif of nuclear factor 7 is required for its association with transcriptional units. Mol Cell Biol 2007; 27:2615 - 2624
- Griffiths S, Dunford RP, Coupland G, Laurie DA. The evolution of CONSTANS-like gene families in barley, rice, and Arabidopsis. Plant Physiol 2003; 131:1855 - 1867
- Wenkel S, Turck F, Singer K, Gissot L, Le Gourrierec J, Samach A, Coupland G. CONSTANS and the CCAAT box binding complex share a functionally important domain and interact to regulate flowering of Arabidopsis. Plant Cell 2006; 18:2971 - 2984
- Datta S, Hettiarachchi GH, Deng XW, Holm M. Arabidopsis CONSTANS-LIKE3 is a positive regulator of red light signaling and root growth. Plant Cell 2006; 18:70 - 84
- Holm M, Hardtke CS, Gaudet R, Deng XW. Identification of a structural motif that confers specific interaction with the WD40 repeat domain of Arabidopsis COP1. EMBO J 2001; 20:118 - 127
- Laubinger S, Marchal V, Le Gourrierec J, Wenkel S, Adrian J, Jang S, Kulajta C, Braun H, Coupland G, Hoecker U. Arabidopsis SPA proteins regulate photoperiodic flowering and interact with the floral inducer CONSTANS to regulate its stability. Development 2006; 133:3213 - 3222