Abstract
Reactive oxygen species (ROS) have been repeatedly implicated as cellular second messengers important in the modulation of almost every ontogenic phase of plant development, from seedling to cell death. In all of these processes, ROS production and detoxification are highly regulated and its levels are kept under tight control, in order to achieve the desired effect at the cellular or plant level. Micropropagated Vitis vinifera L. transferred to ex vitro growth under increased irradiance gave an additional insight into ROS signalling by integrating stress produced hydrogen peroxide (H2O2) into normal signalling pathways with distinctive effects critical for the survival, growth and development of these plants. Here we discuss in further detail the relevance of these results and propose a model that may explain this phenomenon.
In vitro cultures grow in jellified medium inside closed vessels. The special conditions experienced with this type of culture result in the formation of plants with abnormal morphology, anatomy and physiology.Citation1 Transfer to ex vitro conditions is necessary for the recovery of normal plant development: leaf thickness generally increases, leaf mesophyll progresses in differentiation into palisade and spongy parenchyma, stomatal density decreases and stomatal form changes from circular to elliptical, resulting in an effective regulation of transpiration and the stabilization of water status.Citation2 The most important parameters of this transfer to ex vitro and that ultimately determine plant fate are a high relative humidity and an essential increase in irradiance. Like previously described, this change of conditions provokes a photoinhibition of photosynthetic light reactions and the subsequent production of reactive oxygen species (ROS).Citation1,Citation3
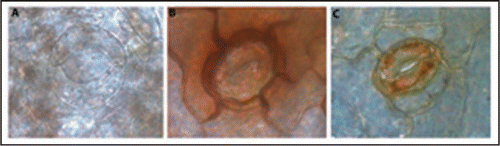
ROS, aside from being by-products of oxidative stress, are well described second messengers in a variety of cellular processes. One of the most interesting of these processes is its functioning as effectors of stomatal closure, in response to abscisic acid (ABA). This is a highly regulated process in which several genes have been implicated (reviewed in ref. Citation4). Using a system that lacked responsiveness to ABA, we were able to decouple ABA from ROS signaling and assess stomatal functioning in a photooxidative stress context.Citation5 In this experiment, micropropagated grapevine evidenced dysfunctional stomata with no ROS accumulation in guard cells prior to transfer to ex vitro. After transfer, a low yet consistent percentage of stomata gained function and were able of a more tight control over pore opening, concomitantly with the accumulation of hydrogen peroxide (H2O2), as depicted in .
There are several factors that contribute to the impairment of ABA signaling in micropropagated grapevine plantlets. Firstly, ABA levels are low before the protruding of roots that only occurs after one week of ex vitro growth. Secondly, the special conditions these plants are grown into potentiate the accumulation of volatile compounds such as ethylene, which is reportedly an antagonist of ABA induced stomatal closure in arabidopsis Citation6 In this experiment, the authors found that ethylene delays stomatal closure by inhibiting the ABA signaling pathway, when comparing the inhibition of ABA induced stomatal closure by ethylene and its precursor ACC (aminocyclopropano-1-carboxylic acid) in wild type, ethylene overproducing mutant (eto1-1) and ethylene insensitive mutants (etr1-1 and ein3-1). Thirdly, ABA mediated stomatal closure is a process that involves an oxidative burst and the active production of ROS and it has also been reported that the state of the antioxidative machinery, namely the major H2O2 scavenger ascorbate, strongly affects stomata opening.Citation7 These authors observed that mid-day light conditions accounted for the lowest ascorbate redox state and the highest level of H2O2, in parallel with a low percentage of open stomata. In addition, plants with increased ascorbate redox state generated by dehydroascorbate reductase overexpression exhibited a decrease of H2O2 in guard cells and had a higher percentage of open stomata. However, an interesting aspect of in vitro culture is that it confers plants a very high ascorbate redox state, certainly due to the high sucrose content in the growth medium.Citation8 Finally and to strengthen the lack of ABA signal, if sufficient amounts of ABA are exogenously applied to in vitro plantlets, ABA mediated stomatal closure does occur to the large majority of stomata.
When plants are taken away from in vitro conditions, ABA is still at very low levels (until one week later), ethylene accumulation at high levels stops occurring (although it could be argued that its effects linger on) and cellular redox state is very high. The most relevant immediate change is the increased irradiance and the subsequent photooxidative stress symptoms that lead to ROS production and accumulation. Superoxide accumulation is observed, at low levels, in all plant tissues as a response to the increased irradiance but H2O2 is detected mainly in veins, wounds and at the stomata level (guard cells and/or surrounding cells).Citation5 This find suggests that there may be a divergent response of the plant to the stressful situation. On one hand, the antioxidative apparatus is functioning effectively and reducing to the maximum the damage caused by ROS production.Citation3 Conversely, H2O2 accumulation is allowed to take place where it may be used as a second messenger in the signaling pathways that are essential for plant survival at this delicate developmental phase.
There have already been described several genes that regulate stomatal aperture independently of ABA. AtMYB61 for instance, a gene encoding a R2R3-MYB family transcription factor, is specifically expressed in guard cells in a manner consistent with involvement in the control of stomatal aperture.Citation9 This regulation of stomatal aperture was proven to be independent of ABA, since the gain or loss of function mutants respond to increasing ABA concentrations as wild type plants. This response is in contrast to ABA-signaling mutants such as ost1 which are much less sensitive to ABA than the wild type.Citation10
The molecular mechanisms that mediate this integration of stress produced ROS into the normal cellular signaling pathways are still elusive but this area should prove interesting for future research, giving additional insight on this novel aspect of stress response.
Figures and Tables
Figure 1 Progression of stomatal functioning over the first week of ex vitro growth (x630). (A) Dysfunctional stomata with no H2O2 accumulation (day 0). (B) Partially functional stomata with H2O2 accumulation on surrounding cells (day 2–4). (C) Functional stomata with H2O2 accumulation on guard cells (day 6–7). Photos are representative of each time point.
Addendum to:
References
- Carvalho LC, Osório ML, Chaves MM, Amâncio S. Chlorophyll fluorescence as an indicator of photosynthetic functioning of in vitro grapevine and chestnut plantlets under ex vitro acclimatization. Plant Cell Tiss Org Cult 2001; 67:271 - 280
- Pospišilová J, Tichá I, Kadlecek P, Haisel D, Plzáková Š. Acclimatization of micropropagated plants to ex vitro conditions. Biol Plant 1999; 42:481 - 497
- Carvalho LC, Vilela BJ, Vidigal P, Mullineaux PM, Amâncio S. Activation of the ascorbate-glutathione cycle is an early response of micropropagated Vitis vinifera L. explants transferred to ex vitro. Int J Plant Sci 2006; 167:759 - 770
- Schroeder J, Allen G, Hugouvieux V, Kwak J, Waner D. Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol 2001; 52:626 - 658
- Vilela BJ, Carvalho LC, Ferreira J, Amâncio S. Gain of function of stomatal movements in rooting Vitis vinifera L. plants: regulation by H2O2 is independent of ABA before the protruding of roots. Plant Cell Rep 2007; 26:2149 - 2157
- Tanaka Y, Sano T, Tamaoki M, Nakajima N, Kondo N, Hasezawa S. Ethylene inhibits abscisic acid-induced stomatal closure in Arabidopsis. Plant Physiol 2005; 138:2337 - 2343
- Chen Z, Gallie DR. The ascorbic acid redox state controls guard cell signaling and stomatal movement. Plant Cell 2004; 16:1143 - 1162
- Conklin PL. Recent advances in the role and biosynthesis of ascorbic acid in plants. Plant Cell Environ 2001; 24:383 - 394
- Liang YK, Dubos C, Dodd IC, Holroyd GH, Hetherington AM, Campbell MH. AtMYB61, an R2R3-MYB transcription factor controlling stomatal aperture in Arabidopsis thaliana. Current Biology 2005; 15:1201 - 1206
- Mustilli AC, Merlot S, Vavasseur A, Fenzi F, Giraudat J. Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 2002; 14:3089 - 3099