Abstract
Bacterial effectors are double-edged swords that enhance bacterial virulence in susceptible plants while trigger resistance in plants carrying cognate resistance proteins. A well-known example of this is Pseudomonas syringae protein AvrPto that is delivered into plant cells through the type III secretion system. AvrPto inhibits immune responses in Arabidopsis plants but triggers resistance in some tomato plants carrying cognate resistance proteins Pto, a serine/threonine kinase, and Prf, a nucleotide-binding leucine-rich repeat protein. In a recent structural study we showed that AvrPto is an inhibitor of the Pto protein kinase. Because Pto closely resemble the kinase domain of receptor kinases, which include pattern recognition receptors (PRRs) crucial for plants to detect invading pathogens, we tested the possibility that PRRs such as FLS2 and EFR are targeted by AvrPto in susceptible plants. Indeed, AvrPto is capable of binding the FLS2 and EFR kinases to block plant immune responses when expressed in protoplasts. In Arabidopsis plants containing FLS2, the P. syringae strain lacking avrPto is compromised in its ability to multiply. However, the defect of the avrPto-deletion strain was alleviated in fls2 plants, indicating a role of AvrPto in overcoming FLS2-mediated resistance. Interestingly, the FLS2-AvrPto and Pto-AvrPto interactions share significant similarity, raising the tantalizing possibility that Pto has evolved as a molecular decoy of the intended targets of AvrPto.
Plants constantly face the threat of potential pathogens. The resistance or disease outcomes are, to large extent, determined by the interplay between the immune systems in the plant and virulence systems possessed by the pathogen. An important layer of plant immunity is PTI, which is activated when plants detect PAMPs originated from plant-associating microbes.Citation1 This immunity is rendered ineffective when plants are challenged with virulent pathogens. For example, a number of P. syringae TTSS effectors are now known to inhibit plant PTI.Citation2,Citation3 Some plants contain NB-LRR proteins that directly or indirectly recognize some of the effectors to initiate ETI, a powerful defense system.Citation4,Citation5 Continued plant-pathogen co-evolution has enabled some pathogen to acquire effectors that specifically overcome ETI.Citation5 Thus, resistance occurs when PTI or ETI is successfully activated, whereas disease ensues often when plant immunity is inhibited by pathogen effectors. Therefore, understanding how pathogen effectors function in inhibiting and triggering of plant defenses has been a major focus of the field.
PTI is initiated when PRRs recognize PAMPs at the plant cell surface.Citation1 Plant PRRs known to detect pathogenic bacterial PAMPs include two receptor kinases, FLS2 and EFR. FLS2 recognizes a conserved N-terminal peptide of flagellin designated flg22, whereas EFR detects a conserved peptide from the elongation factor EF-Tu called elf18.Citation6,Citation7 The binding of ligands to FLS2 and EFR stimulates a cytoplasmic signaling pathway to trigger defenses. For example, the binding of flg22 to FLS2 induces its dimerization with another receptor-like kinase BAK1.Citation8,Citation9 Downstream, at least two MAP kinase cascades are activated to positively or negatively regulate PTI responses, which include transcriptional activation of gene expression, oxidative burst, and cell wall-based defenses.Citation1
The Pseudomonas syringae effector AvrPto is directly recognized by the serine/threonine kinase Pto carried by some tomato plants to activate ETI specified by Prf, a NB-LRR protein.Citation10,Citation11 In Arabidopsis and susceptible tomato plants lacking Pto or Prf, AvrPto inhibits PTI and enhances bacterial virulence, but the virulence target(s) remained to be identified.Citation12–Citation14
In order to gain biochemical insights of the AvrPto protein, we solved the crystal structure of the Pto-AvrPto protein complex.Citation15 Interestingly AvrPto binds Pto as a pseudosubstrate. In vitro analysis confirmed that AvrPto is an inhibitor of the Pto kinase. Sequence alignment and computer modeling suggested that Pto is structurally similar to the kinase domain of receptor kinases.Citation16 We reasoned that AvrPto might have evolved as an inhibitor of receptor kinases. To test this possibility, we applied four different methods to determine if AvrPto is able to interact with FLS2 and EFR in vitro and in the protoplast. Protein pull-down and BIAcore surface plasmon resonance assays showed that AvrPto indeed interacts with the kinase domain of FLS2 and EFR in vitro. BiFC and co-immunoprecipitation (co-IP) assays showed that AvrPto is capable of interacting with the full-length FLS2 and EFR in the protoplast. Because FLS2 dimerizes with BAK1 when induced by flg22, the observed AvrPto-FLS2 interaction in the protoplast may be indirectly mediated by BAK1. To rule-out this possibility, we performed the co-IP assay in the absence of flg22 treatment and observed normal interaction. Furthermore, co-IP assays detected normal AvrPto-FLS2 interaction in bak1 null mutant protoplasts, indicating that the observed interaction is BAK1-independent. Together with the in vitro protein-protein interaction data, these results demonstrated that AvrPto can directly interact with FLS2 and EFR kinase domain in vivo.
If FLS2 is a major target of AvrPto, P. syringae bacteria lacking avrPto may display reduced virulence on Arabidopsis plants carrying FLS2. This deficiency should be alleviated in Arabidopsis plants lacking FLS2. Spray inoculation of wild-type (WT) and fls2 mutant Arabidopsis plants with the P. syringae strain DC3000, which carries avrPto, and the derivative strain lacking avrPto showed that this was indeed the case. The ΔavrPto strain grew to a lower level than did the DC3000 strain on WT Arabidopsis plants. On fls2 mutant plants, both strains grew to a similarly high level. AvrPto is similarly capable of interacting with the tomato flg22 receptor LeFLS2, and the ability to interact with LeFLS2 correlated with the virulence activity of AvrPto on susceptible tomato plants. These results demonstrated that the observed FLS2-AvrPto interaction is relevant to the immune suppression activity and virulence function of AvrPto.
We also tested the similarity of Pto-AvrPto and FLS2-AvrPto interactions. Both interactions required the AvrPto Y89 residue that makes direct contact with the kinase. The interaction also requires a functional ATP binding site, suggesting that autophosphorylation of the kinases are required for the recognition by AvrPto.Citation15 The AvrPto I96 is required for interacting with Pto, and this residue is partially required for interaction with FLS2. Furthermore, Pto is capable of competing with FLS2 for binding to AvrPto. These results indicate that the Pto-AvrPto and FLS2-AvrPto interactions are at least partially similar and involve overlapping interaction surfaces, suggesting that Pto may be mimicking FLS2 for AvrPto recognition.
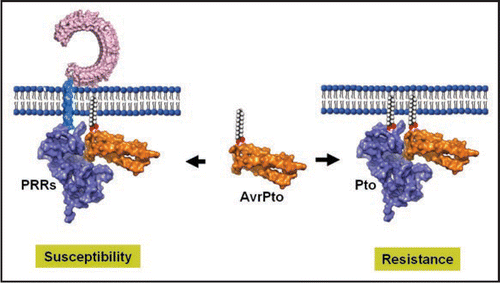
It has been proposed that Pto is a virulence target of AvrPto, and Prf acts by “guarding” the Pto protein (the guardee), allowing plant cells to sense the virulence activity of the bacterial effector and activate immune responses.Citation17 This “guard model” has since been adopted to explain the recognition of a number of indirect recognitions between resistance proteins and pathogen effectors. However, the “guardees” identified to-date do not appear to fulfill a role of virulence targets. Pto is not required for the virulence function of AvrPto in tomato or Arabidopsis plants, indicating that other proteins are virulence targets.Citation12 We propose an alternative model in which Pto has been adopted by plants to “deceive” the pathogen by mimicking the kinase domain of PRRs, the intended virulence targets for AvrPto (). The binding of AvrPto to Pto does not block PAMP-induced immune responses. Instead, it enables plant cells to sense the intruder and trigger ETI through Prf. Similarly, the tomato cysteine protease Rcr3, Arabidopsis RPM1-interacting protein RIN4, and Arabidopsis protein kinase PBS1 all mediate indirect recognitions between pathogen effectors and resistance proteins.Citation18–Citation22 None of these proteins is required for the virulence function of the corresponding pathogen effectors. In each case, there exist homologous host proteins that may be targeted by the effectors for virulence.Citation18,Citation23 There is a formal possibility that some of these NB-LRR proteins guard the decoy of virulence targets.
Abbreviations
| PRRs | = | pattern recognition receptors |
| TTSS | = | type III secretion system |
| PAMPs | = | pathogen-associated molecular patterns |
| PTI | = | PAMP-triggered immunity |
| ETI | = | effector-triggered immunity |
| NB-LRR proteins | = | nucleotide-binding leucine-rich repeat proteins |
Figures and Tables
Figure 1 Model for PTI-inhibition and ETI-induction by AvrPto. In susceptible plants, AvrPto binds PRRs including FLS2 and EFR. This blocks PTI activation and enhances host susceptibility to the bacterium. In resistant plants, Pto is evolved to mimic the kinase domain of PRRs and binds AvrPto to trigger ETI, resulting in a resistance outcome. The Pto-AvrPto structure is adopted from Xing et al.Citation1 The blue rod connecting the LRR and kinase domains of FLS2 represents the transmembrane domain, whereas the rods on Pto and AvrPto denote myristic acid that targets these proteins to plasma membranes.
Addendum to:
References
- Bittel P, Robatzek S. Microbe-associated molecular patterns (MAMPs) probe plant immunity. Curr Opin Plant Biol 2007; 10:335 - 341
- Abramovitch RB, Anderson JC, Martin GB. Bacterial elicitation and evasion of plant innate immunity. Nat Rev Mol Cell Biol 2006; 7:601 - 611
- da Cunha L, Sreerekha MV, Mackey D. Defense suppression by virulence effectors of bacterial phytopathogens. Curr Opin Plant Biol 2007; 10:349 - 357
- Chisholm ST, Coaker G, Day B, Staskawicz BJ. Host-microbe interactions: shaping the evolution of the plant immune response. Cell 2006; 124:803 - 814
- Jones JD, Dangl JL. The plant immune system. Nature 2006; 444:323 - 329
- Chinchilla D, Bauer Z, Regenass M, Boller T, Felix G. The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception. Plant Cell 2006; 18:465 - 476
- Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JD, Boller T, Felix G. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 2006; 125:749 - 760
- Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nurnberger T, Jones JD, Felix G, Boller T. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 2007; 448:497 - 500
- Heese A, Hann DR, Gimenez Ibanez S, Jones AM, He K, Li J, Schroeder JI, Peck SC, Rathjen JP. The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci USA 2007; 104:12217 - 12222
- Tang X, Frederick RD, Zhou J, Halterman DA, Jia Y, Martin GB. Initiation of plant disease resistance by physical interaction of AvrPto and Pto kinase. Science 1996; 274:2060 - 2063
- Salmeron JM, Oldroyd GED, Rommens CMT, Scofield SR, Kim HS, Lavelle DT, Dahlbeck D, Staskawicz BJ. Tomato Prf is a member of the leucine-rich-repeat class of plant disease resistance genes and lies embedded within the Pto kinase gene cluster. Cell 1996; 86:123 - 133
- Shan L, He P, Zhou JM, Tang X. A cluster of mutations disrupt the avirulence but not the virulence function of AvrPto. Mol Plant Microbe Interact 2000; 13:592 - 598
- Hauck P, Thilmony R, He SY. A Pseudomonas syringae type III effector suppresses cell wall-based extracellular defense in susceptible Arabidopsis plants. Proc Natl Acad Sci USA 2003; 100:8577 - 8582
- He P, Shan L, Lin NC, Martin GB, Kemmerling B, Nürnberger T, Sheen J. Specific bacterial suppressors of MAMP signaling upstream of MAPKKK in Arabidopsis innate immunity. Cell 2006; 125:535 - 563
- Xing W, Zou Y, Liu Q, Liu J, Luo X, Huang Q, She C, Zhu L, Bi R, Hao Q, Wu JW, Zhou JM, Chai J. The structural basis for activation of plant immunity by bacterial effector protein AvrPto. Nature 2007; 449:243 - 247
- Xiang T, Zong N, Yan Zou, Wu Y, Zhang J, Xing W, Li Y, Tang X, Zhu L, Chai J, Zhou J. Pseudomonas syringae effector AvrPto blocks innate immunity by targeting receptor kinases. Curr Biol 2008; 18:74 - 80
- van der Biezen EA, Jones JDG. Plant disease-resistance proteins and the gene-for-gene concept. Trends Biochem Sci 1998; 23:454 - 456
- Rooney HC, Van't Klooster JW, van der Hoorn RA, Joosten MH, Jones JD, de Wit PJ. Cladosporium Avr2 inhibits tomato Rcr3 protease required for Cf-2-dependent disease resistance. Science 2005; 308:1783 - 1786
- Mackey D, Holt BF 3rd, Wiig A, Dangl JL. RIN4 interacts with Pseudomonas syringae type III effector molecules and is required for RPM1-mediated resistance in Arabidopsis. Cell 2002; 108:743 - 754
- Axtell MJ, Staskawicz BJ. Initiation of RPS2-specified disease resistance in Arabidopsis is coupled to the AvrRpt2-directed elimination of RIN4. Cell 2003; 112:369 - 377
- Mackey D, Belkhadir Y, Alonso JM, Ecker JR, Dangl JL. Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS2-mediated resistance. Cell 2003; 112:379 - 389
- Shao F, Golstein C, Ade J, Stoutemyer M, Dixon JE, Innes RW. Cleavage of Arabidopsis PBS1 by a bacterial type III effector. Science 2003; 301:1230 - 1233
- Kim HS, Desveaux D, Singer AU, Patel P, Sondek J, Dangl JL. The Pseudomonas syringae effector AvrRpt2 cleaves its C-terminally acylated target, RIN4, from Arabidopsis membranes to block RPM1 activation. Proc Natl Acad Sci USA 2005; 102:6496 - 6501