Abstract
Endothermic heating of floral tissues and even thermoregulation is known to occur in a number of plant species across a wide taxonomic range. The mechanisms by which flowers heat, however, are only just beginning to be understood, and even less is known about how heating is regulated in response to changes in ambient temperature. We have recently demonstrated that the alternative pathway of respiration, in which the alternative oxidase (AOX) rather than cytochrome C (COX) acts as terminal electron acceptor, is responsible for heat generation in one thermoregulating species, the sacred lotus (Nelumbo nucifera). In the March issue of the Journal of Experimental Botany we further demonstrated that AOX-mediated heat production in this species is regulated at both the level of gene expression and also post-translationally. Similarly, AOX has also been implicated in heat production in other thermogenic species. In this addendum we discuss the central role of AOX in heat production and how post-translational mechanisms may provide the fine control necessary for thermoregulation.
Addendum to: Grant NM, Miller RE, Watling JR, Robinson SA. Synchronicity of thermogenic activity, alternative pathway respiratory flux, AOX protein content, and carbohydrates in receptacle tissues of sacred lotus during floral development. J Exp Bot 2008; 59:705-14.
Heating in Plants
The internal generation of heat to maintain body temperature, endothermy, is usually associated with birds and mammals, however, it also occurs in some flowers. Endothermy in flowers is probably an adaptation to enhance rates of pollination through release of attractant chemicals,Citation1 or providing a heat reward to pollinating insects;Citation2 although it may also be associated with floral development or protection from low temperatures.Citation3 A diverse range of plants display endothermy,Citation4 and a small number of these are capable of thermoregulation, that is, sensing external temperature changes and regulating heat production at the cellular level to maintain tissue temperature within a narrow range (). Two thermoregulating plants that have been studied in some detail are the sacred lotus, Nelumbo nucifera (a eudicot), and Symplocarpus renifolius (a monocot). Thermoregulation in these two unrelated species is an interesting example of convergent evolution, and a fascinating physiological phenomenon that poses a number of questions. For example, how do plants regulate heat production without the complex neural and hormonal systems found in birds and mammals? As a first step to answering this question our group has been investigating the mechanisms by which plants generate heat.
Endothermic animals generate heat metabolically by ‘burning’ fats and sugars during respiration. In contrast, respiration rates in most plants are too low to generate sufficient heat to warm their tissues. Plants, however, have at least two mechanisms by which mitochondrial electron transport can be uncoupled from ATP regeneration, thereby allowing the energy to instead be used for heat generation. The first of these involves the alternative oxidase (AOX), or cyanide-resistant pathway, which occurs in all plants as well as in fungi, prokaryotes and many animal taxa.5 The second involves uncoupling proteins (UCP), which are known to occur in many eukaryotes.Citation6
The AOX is a nuclear encoded, integral membrane protein that is present as a homodimer in the inner mitochondrial membrane of all plants.Citation7 Electron transport to oxygen via the AOX branches from the main mitochondrial electron transport chain at ubiquinone, instead of passing to oxygen via the COX pathway, and thus is largely uncoupled from ATP production, with energy being released as heat.Citation8 Similarly, plant UCPs are also nuclear encoded, integral membrane proteins that exist as homodimers in the inner mitochondrial membrane.Citation6,Citation9 Their mode of action differs from AOX however, in that UCPs act by dissipating the proton gradient that is generated by electron transport, resulting in high (uncoupled) electron fluxes to oxygen through COX.Citation6 In most plants the activity of these two pathways is quite low and their function is not thermogenesis, but is probably linked to preventing cellular oxidative stress.Citation7 Recent evidence suggests that the main role of UCPs in plants maybe to improve photosynthetic efficiency.Citation10 The existence of at least two potential heat generating pathways, and technical difficulties in quantifying flux through either, have meant that the mechanism(s) of heating in thermogenic flowers have so far remained a matter of speculation. Recently, however, our group has applied a combination of stable oxygen isotope and standard protein quantification techniques to uncover the heating mechanism in the sacred lotus.Citation11,Citation12
The AOX Plays a Central Role in Heating in Sacred Lotus
As mentioned above, both AOX and UCPs may play a role in plant thermogenesis. To determine the extent to which either may be involved in heating in the sacred lotus we have quantified in vivo electron flux through both the AOX and COX pathways using online stable oxygen isotope discrimination techniques. Our studies have found that AOX flux increases significantly with heating and can account for up to 93% of electron transport in the hottest flowers, while electron flux to COX does not change with heating, suggesting that UCPs are not involved in heat generation in this species.Citation11
Our recent paper has provided further evidence of a central role for AOX in thermogenesis in sacred lotus.Citation12 In this paper we demonstrated that the onset of thermogenic activity in sacred lotus flowers coincides with a rapid, 10-fold increase in AOX protein content, relative to pre-thermogenic tissues. This dramatic change in AOX content occurs over a 1–2 d period during the well-defined developmental sequence in sacred lotus flowers. In contrast, there was no change in the amount of COX protein over the same developmental sequence,Citation12 and so far we have been unable to detect any UCP protein in floral tissues of sacred lotus (unpublished data). Together these data suggest that coarse regulation of heating in this species occurs at the level of AOX gene expression, however, they do not explain how AOX activity is regulated during the thermogenic stages to maintain a constant tissue temperature in the face of changing ambient temperature.
In attempting to answer this last question, we have shown that there is a strong relationship between electron flux through AOX and the amount of heating in thermogenic tissues of sacred lotus.Citation11,Citation12 That is, as air temperature declines and more heat is needed to maintain tissue temperature, AOX flux also increases. We have also demonstrated, however, that there is no change in the amount of AOX protein in floral tissues during the thermogenic stages.Citation12 In other words, regulation of heat production via AOX flux in thermogenic tissues of sacred lotus is post-translational, and does not involve changes in the amount of AOX protein. Thus, understanding how heating is regulated in this particular thermoregulating species requires knowledge of the post-translational regulation of AOX.
A significant amount is known about post-translational regulation of AOX from non-thermogenic plant tissues, where it has been shown to be mediated by a number of mechanisms, including: the redox state of the ubiquinone pool,Citation13 whether AOX is present in dimeric (inactive) or monomeric (active) form,Citation14 and interaction with α-keto acids (e.g., pyruvate), that stimulate activity of monomeric AOX.Citation15 It is also known that isoforms of the AOX protein with amino acid substitutions at critical sites (e.g., cysteine residues 1 & 2) vary in their response to these regulatory factors.Citation16,Citation17 We are currently investigating post-translational regulation of AOX in thermogenic tissues of sacred lotus and our preliminary data suggest that it could have amino acid substitutions that alter the way it is post-translationally regulated, relative to AOX from non-thermogenic tissues in this and other plants (unpublished data).
What About other Thermogenic Plants?
The sacred lotus remains the only thermogenic species to date in which electron flux through the AOX and COX pathways has been quantified in vivo. Flux control analysis, however, also supports a central role for AOX during thermogenesis in the monocot Arum maculatum, with COX again playing an insignificant role, suggesting that UCP is similarly not involved in heating in this species.Citation18 By contrast, there is significant biochemical and molecular evidence from another thermogenic species, Symplocarpus renifolius, indicating the involvement of both AOX and UCP in heating.Citation19 Thus, while a central role for the AOX has been established for sacred lotus and probably A. maculatum, it is possible that in some thermogenic plants UCPs may also be involved.
Figures and Tables
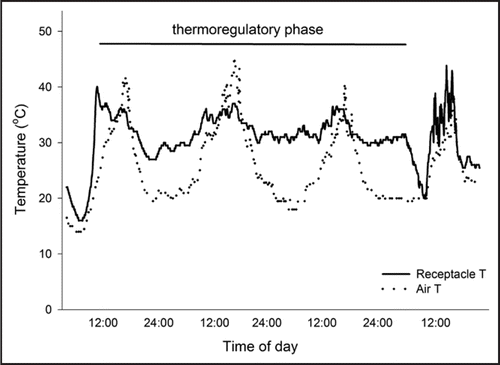
Figure 1 Changes in air temperature (dotted line) and receptacle temperature (solid line) for sacred lotus, Nelumbo nucifera, over the 2–3 d thermoregulatory phase of floral development. Floral development occurs over 4–5 d and during the thermoregulatory phase receptacle temperature is maintained at close to 32°C, while air temperature can vary from 8–45°C.Citation4,Citation12
Acknowledgements
This work was supported by the Australian Research Council (DP 0451617).
Addendum to:
References
- Meeuse BJD. Thermogenic respiration in aroids. Ann Rev Plant Physiol Plant Mol Biol 1975; 26:117 - 126
- Seymour RS, White CR, Gibernau M. Heat reward for insect pollinators. Nature 2003; 426:243 - 244
- Knutson RM. Heat production and temperature regulation in eastern skunk cabbage. Science 1974; 186:746 - 747
- Seymour RS. Biophysics and physiology of temperature regulation in thermogenic flowers. Biosci Rep 2001; 21:223 - 236
- McDonald AE, Vanlerberghe AE. Origins, evolutionary history and taxonomic distribution of alternative oxidase and plastoquinol terminal oxidase. Comp Biochem Physiol D-Genomics & Proteomics 2006; 1:357 - 364
- Vercesi AE, Borecky J, Maia IdG, Arruda P, Cuccovia IM, Chaimovich H. Plant uncoupling mitochondrial proteins. Ann Rev Plant Biol 2006; 57:383 - 404
- Vanlerberghe GC, McIntosh L. Alternative oxidase: From gene to function. Ann Rev Plant Physiol Plant Mol Biol 1997; 48:703 - 734
- Moore AL, Siedow JN. The regulation and nature of the cyanide-resistant alternative oxidase of plant mitochondria. Biochim Biophys Acta 1991; 1059:121 - 140
- Hourton-Cabassa C, Rita Matos A, Zachowski A, Moreau F. The plant uncoupling protein homologues: a new family of energy-dissipating proteins in plant mitochondria. Plant Physiol Biochem 2004; 283 - 290
- Sweetlove LJ, Lytochenko A, Morgan M, Nunes-Nesi A, Taylor NL, Baxter CJ, Fernie AR. Mitochondrial uncoupling protein is required for efficient photosynthesis. Proc Natl Acad Sci USA 2006; 103:19587 - 19592
- Watling JR, Robinson SA, Seymour R. Contribution of the alternative pathway to respiration during thermogenesis in flowers of the Sacred lotus, Nelumbo nucifera. Plant Physiol 2006; 140:1367 - 1373
- Grant NM, Miller RE, Watling JR, Robinson SA. Synchronicity of thermogenic activity, alternative pathway respiratory flux, AOX protein content and carbohydrates in receptacle tissues of sacred lotus during floral development. J Exp Bot 2008; 59:705 - 714
- Dry IB, Moore AL, Day DA, Wiskich JT. Regulation of alternative pathway activity in mitochondria; non-linear relationship between electron flux and the redox poise of the quinone pool. Arch Biochem Biophys 1989; 273:148 - 157
- Umbach AL, Siedow JN. Covalent and noncovalent dimers of the cyanide-resistant alternative oxidase protein in higher plant mitochondria and their relationship to enzyme activity. Plant Physiol 1993; 103:845 - 854
- Vanlerberghe GC, Yip JYH, Parsons HL. In organello and in vivo evidence of the importance of the regulatory sulfhydryl/disulfide system and pyruvate for alternative oxidase activity in tobacco. Plant Physiol 1999; 121:793 - 803
- Crichton PG, Affourti C, Albury MS, Carré JE, Moore AL. Constitutive activity of Sauromatum guttatum alternative oxidase in Schizosaccharomyces pombe implicates residues in addition to conserved cysteines in α-keto acid activation. FEBS Letts 2005; 579:331 - 336
- Umbach AL, Ng VS, Siedow JN. Regulation of plant alternative oxidase activity: A tale of two cysteines. Biochim Biophys Acta 2006; 1757:135 - 142
- Wagner AM, Krab K, Wagner MJ, Moore AL. Regulation of thermogenesis in flowering Araceae: The role of the alternative oxidase. Biochim Biophys Acta 2008; In press http://dx.doi.org/10.1016/j.bbabio.2008.04.001
- Onda Y, Kato Y, Abe Y, Ito T, Morohashi M, Ito Y, Ichikawa M, Matsukawa K, Kakizaki Y, Koiwa H, Ito K. Functional coexpression of the mitochondrial alternative oxidase and uncoupling protein underlies thermoregulation in the thermogenic florets of skunk cabbage. Plant Physiol 2008; 146:636 - 645