Abstract
Root mucilage is gelatinous polysaccharide-containing material exuded from the outer layers of the root cap. Although mucilage has been suggested to play several roles in plant growth, its role in mineral uptake has not been well understood. Melastoma malabathricum L. is an aluminum (Al) accumulator growing in tropical acid soils. This species accumulates more than 10 mg Al g-1 DW in leaves and roots. Root mucilage is generally known to immobilize metal cations such as Al in the rhizosphere. However, we found that roots of M. malabathricum exuded large amounts of mucilage. Using the Zea mays L. mucilage as a control, we have recently shown that mucilage of M. malabathricum has unique physical and chemical characteristics, and facilitates Al uptake in this species. Since M. malabathricum cannot grow well in Al-deficient soil (nonacid soils), this species might have developed a mechanism for Al acquisition. We have also discussed the reason for this species' requirement of Al, a nonessential element.
Addendum to: Watanabe T, Misawa S, Hiradate S, Osaki M. Characterization of root mucilage from Melastoma malabathricum, with emphasis on its roles in aluminum accumulation. New Phytol 2008;178:581-9.
Plant roots exude various chemical compounds. Root mucilage is a gelatinous high molecular weight compound, consisting mainly of polysaccharides, and is exuded from the outer layers of the root cap. One of the suggested roles of mucilage in plant growth is detoxification (fixation) of toxic metal cations, including Al in acidic soils.Citation1 Polysaccharides in mucilage contain uronic acids,Citation2 the carboxyl groups of which might adsorb and inactivate Al in the rhizosphere.Citation3 However, Li et al.,Citation4 suggested that the contribution of mucilage to Al inactivation in the rhizosphere of Zea mays L. is almost negligible owing to its small amount.
Melastoma malabathricum L. is an Al accumulator growing in tropical acidic soils and is one of the dominant woody species growing in acid sulfate soils in tropical Asia, Australia and Polynesia. This species accumulates more than 10 mg Al g−1 DW in leaves and roots.Citation5 It is known that the growth of non-essential element-accumulating plants is often enhanced by the application of the accumulated element (e.g., Na in a Na accumulator plant). The growth of M. malabathricum is enhanced by Al application under hydroponic conditions.Citation6 It has been predicted that this growth enhancement is due to alleviation of iron toxicityCitation7 or a disorder of organic acid metabolism in the absence of Al.Citation8 Recently, we observed that the roots of M. malabathricum exuded large amounts of mucilage under “Al deficient” conditions, raising a question about the role of mucilage in this species.
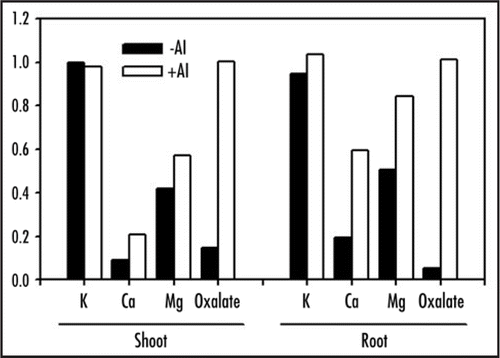
Our recent study indicated that M. malabathricum mucilage facilitates Al uptake in this species.Citation9 Contrastingly, uptake of other cations such as K, Ca and Mg were not affected. To the best of our knowledge, this is the first study to demonstrate that mucilage selectively enhances mineral uptake in plant. To elucidate the factors that were responsible for these characteristics of M. malabathricum mucilage, we compared the physical and chemical properties of M. malabathricum mucilage with that of Z. mays mucilage, which is known to strongly bind to Al.Citation4 The results are summarized in . Critical differences in the physical properties that can affect Al uptake of roots were evaluated. M. malabathricum mucilage has higher adsorption affinities for trivalent cations, whereas Z. mays mucilage has a higher affinity for divalent cations. Moreover, 27Al NMR analysis and bioassay indicated that the binding strength between mucilage and Al was very weak in M. malabathricum mucilage and its ionic activity was nearly equal to inorganic monomeric Al ions.
What is the primary factor behind such special characteristics of Al adsorption in the M. malabathricum mucilage? In general, a cation exchanger with a high charge density exhibits preferences for trivalent cations.Citation10 The cation exchange capacity per unit volume in the M. malabathricum mucilage is nearly twice as high as that in Z. mays mucilage because of its higher proportion of uronic acid (glucuronic acid) (). This high charge density is considered to be the primary reason for higher affinity of the trivalent cation in M. malabathricum mucilage. Conversely, the weak binding strength between mucilage and Al is possibly due to the higher degree of methylation in M. malabathricum mucilage. It has been suggested that like unmethylated polyuronic acid, partly methylated polyuronic acid can form “egg box” junctions;Citation11 however, the binding strength is weak.Citation12 In addition, the lower pH of M. malabathricum mucilage is also responsible for the weak binding strength.
M. malabathricum has evolved in extremely acidic soils. This medium, with a certain level of Al ion, might be suitable for the growth of this species, as opposed to Al-free medium. Recently, we found that water-soluble Ca, Mg and oxalate concentrations in both shoots and roots of M. malabathricum grown hydroponically in the absence of Al were significantly lower than that in the presence of Al (). Irrespective of Al concentration in the medium, M. malabathricum synthesizes a high concentration of oxalate, which is a ligand for Al detoxification in plant tissue.Citation8 The high concentration of oxalate would make insoluble precipitates with Ca and Mg when grown in the absence of Al, inducing severe deficiency of these elements in M. malabathricum. Therefore, mechanisms of Al acquisition are essential for M. malabathricum when growing in Al deficient soils ().
Abbreviations
| Al | = | aluminum |
| NMR | = | nuclear magnetic resonance |
| Gal | = | galactose |
| GlcA | = | glucuronic acid |
| Fuc | = | fucose |
| Xyl | = | xylose |
| ICP-OES | = | inductively coupled plasma-optical emission spectrometry |
Figures and Tables
Figure 1 Characterization of root mucilage in Melastoma malabathricum. (A) Differences in physical and chemical properties of mucilage between M. malabathricum and Zea mays. (B) Hypothetical model of selective adsorption of cation in M. malabathricum and Z. mays. Mucilage of M. malabathricum has high affinity for trivalent cations because of its higher charge density. (C) Hypothetical scheme of Al acquisition in M. malabathricum.
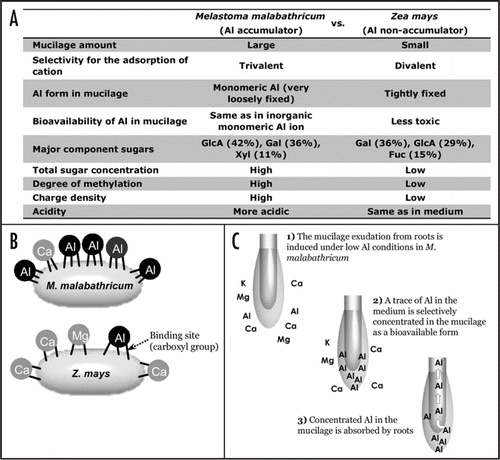
Figure 2 Ratio of water-soluble K, Ca, Mg and oxalate to total concentration in Melastoma malabathricum grown in a nutrient solution with or without 0.5 mM AlCl3 for 30 days. Water soluble K, Ca, Mg and oxalate concentrations were determined by ICP-OES or capillary electrophoresis after extracting with deionized water (lyophilized sample:water = 1:10). Total K, Ca and Mg concentrations were determined by ICP-OES after wet-digestion. Total oxalate concentrations were determined by capillary electrophoresis after extracting with 0.2 M HCl. The standard nutrient solution contained 0.54 mM N (NH4NO3), 32 µM P (NaH2PO4·2H2O), 0.15 mM K (K2SO4:KCl = 1:1), 0.25 mM Ca (CaCl2·2H2O), 0.16 mM Mg (MgSO4·7H2O), 35.8 µM Fe (FeSO4·7H2O), 1.8 µM Mn (MnSO4·4H2O), 9.26 µM B (H3BO3), 0.62 µM Zn (ZnSO4·7H2O), 0.036 µM Cu (CuSO4·5H2O) and 0.01 µM Mo ((NH4)6Mo7O24·4H2O); total SO4 = 0.21 mM. There was no significant difference in total concentrations between the treatments.
Addendum to:
References
- Horst WJ, Wagner A, Marschner H. Mucilage protects root meristems from aluminium injury. Z Pflanzenphysiol 1982; 109:95 - 103
- Moody SF, Clarke AE, Bacic A. Structural analysis of secreted slime from wheat and cowpea roots. Phytochem 1988; 27:2857 - 2861
- Mench M, Morel JL, Guckert A. Metal binding properties of high molecular weight soluble exudates from maize (Zea mays L.) roots. Biol Fertil Soils 1987; 3:165 - 169
- Li XF, Ma JF, Hiradate S, Matsumoto H. Mucilage strongly binds aluminum but does not prevent roots from aluminum injury in Zea mays. Physiol Plant 2000; 108:152 - 160
- Watanabe T, Osaki M, Yoshihara T, Tadano T. Distribution and chemical speciation of aluminum in the Al accumulator plant, Melastoma malabathricum L.. Plant Soil 1998; 201:165 - 173
- Watanabe T, Jansen S, Osaki M. The beneficial effect of aluminium and the role of citrate in Al accumulation in Melastoma malabathricum. New Phytol 2005; 165:773 - 780
- Watanabe T, Osaki M. Role of organic acids in aluminum accumulation and plant growth in Melastoma malabathricum. Tree Physiol 2002; 22:785 - 792
- Watanabe T, Jansen S, Osaki M. Al-Fe interactions and growth enhancement in Melastoma malabathricum and Miscanthus sinensis dominating acid sulphate soils. Plant Cell Environ 2006; 29:2124 - 2132
- Watanabe T, Misawa S, Hiradate S, Osaki M. Characterization of root mucilage from Melastoma malabathricum, with emphasis on its roles in aluminum accumulation. New Phytol 2008; 178:581 - 589
- Falke JJ, Snyder EE, Thatcher KC, Voertler CS. Quantitating and engineering the ion specificity of an EF-hand-like Ca2+ binding. Biochemistry 1991; 30:8690 - 8697
- Grant GT, Morris ER, Rees DA. Biological interactions between polysaccharides and divalent cations: the egg-box model. FEBS Lett 1973; 32:195 - 198
- Carpita NC, McCann MC. Buchanan BB, Gruissem W, Jones RL. Chapter 2. The cell wall. Biochemistry & Molecular Biology of Plants 2000; Rockville American Society of Plant Physiologists 52 - 108