Abstract
In addition to the mechanical forces of the external environment, the individual plant cell is also subject to multiple subtle biophysical forces that arise from neighboring cell growth and division within the tissue. To maintain a normal cell shape and division pattern, the plant cell is proposed to have the ability to sense and respond to repetitive subtle mechanical stimulations via nuclear-directed migration. It has been demonstrated that the nucleus is alert and highly sensitive to repetitive mechanical stimulations. Furthermore, the cytoplasm reacts to local mechanical stimulation in a compartmentalized fashion. The nucleus therefore plays a role as a chief organizer and active defender in response to mechanical stimulation. This finding provides new insight on the role of mechanical stimulation in regulating cell division and the consequent spatial positioning and shape of cells inside tissues. The finding also revealed that it necessitates further study into the reason for cytoplasmic functional compartmentalization in response to simulation in the context of cell evolution.
Introduction
Mechanical stimulation is a common assault on plants in nature; typical and widely known phenomena of plant movement in response to stimulation are known as thigmonasty (rapid, reversible and directionless response to touch), thigmotropism (directional response to touch), and thigmomorphogenesis (altered growth pattern in response to touch and wind).Citation1,Citation2 However, it is largely unknown how an individual cell senses mechanical stimulation and transfers the signal to other cells. Additionally, it is unknown how stimulation results in cell division or how specific structures are formed in response to mechanical stimulations.
Lintilhac and VeseckyCitation3 have shown that external compressive forces induce the alignment of division planes in plant tissues grown in vitro. Although they did not describe the behavior of individual cells in response to mechanical stimulations, their experiments revealed two important clues for understanding the mechanism of the internal cell response to such stimulations. First, the signals of mechanical stimulations can be transferred from cell to cell and reach the cells deep inside the tissue. Additionally, a cluster of cells is able to respond to a signal as a whole. Second, mechanical stimulation can regulate the orientation of cell divisions. This might form the basis of morphogenetic alterations in response to persistent or frequent mechanical stimulations.
The whole plant body, comprising thousands of millions of cells, can be viewed as a huge system. In fact, within the system, mechanical stimulations may be generated frequently, for example, the pressure exerted on the lower cell layers from the top cells due to gravity and the pressure that arises by a neighboring cell's expansion or division.Citation4 Plant cells are accustomed to these gentle pressures because they develop gradually, and are constantly received from adjacent cells in all directions. Based on previous findings, it is reasonable to propose that such mechanical pressures play an important role in regulating cell division and development in plant tissues, thereby maintaining a cell shape and division pattern that is consistent with normal morphogenesis of certain plant organs. Thus, it is necessary that individual, internal cells are able to sense and respond to these multiple and subtle mechanical stimulations. However, although the ability of an epidermal cell to sense and respond to external mechanical forces is widely accepted, whether an individual internal plant cell can sense repetitive mechanical stimulations and the mechanisms of plant cell responses have not been largely illustrated until now. Therefore, in the context of regulating and maintaining normal morphogenesis, it is of utmost importance to understand the behavior of an internal cell in response to multiple and subtle mechanical stimulations.
The Nucleus Plays an Active Role in Responding to Mechanical Stimulations
One of the most famous subcellular changes upon external mechanical stimulation is nuclear movement to the wounding site, which was termed traumatotactic nuclear migration by Nagai,Citation5 and was subsequently reported by many researchers.Citation6–Citation8 Even isolated plant nuclei could act as mechanical and thermal sensors.Citation9 Similarly, nuclear movement is a common phenomenon during cell development in various tissues. Cells within tissues adjust the nuclear position to regulate symmetric/asymmetric cell division and successive cell differentiation and to control cell fate such as in the process of stomatal formation.Citation7,Citation10 The final location of the nucleus results from the accumulated effects of repetitive stimulations that adjust nuclear orientation and movement. Thus, we propose that the nucleus of a plant cell is also active in sensing mechanical stimulation and that nuclear migration could be used as a marker to evaluate the cell's sensitivity to repetitive and subtle stimulations.
To explore nuclear capacity and sensitivity to rapidly changing signals, we carried out repetitive mechanical stimulations on single tobacco leaf vein hair cells either locally or at a distance; these cells are transparent and the nucleus is the only visible organelle under optical microscopy. Our results indicated that the nucleus retains high sensitivity to repetitive local mechanical stimulations; the nucleus responded to a second stimulation without loss of sensitivity and velocity, regardless of whether it was in a resting or moving state.Citation11 This finding indicates that plant cells have the ability to sense and respond to repetitive external mechanical stimulations. Therefore, it is possible that they react to continuous biophysical forces within tissues. To confirm that this is an inherent ability of plant cells, we recently developed a convenient experimental system to test how cells derived from higher plant internal tissues respond to mechanical stimulation. Short-term in vitro culture of tobacco ovules derived from the parenchyma tissue of the ovule funicle led to the generation of bar-shaped cells. These cells were still connected to the mother tissue and remained almost undifferentiated. We applied local mechanical stimulations to the internal cells and found that although the nucleus of an internal cell did not react to multiple mechanical stimulations as fast as that of epidermal cells, it quickly responded and moved to the stimulation site.Citation12 These experiments confirmed the active role of the nucleus in response to mechanical stimulations. Because the nuclear position is closely related to the future division plane,Citation13 the adjustment of nuclear location by variation in mechanical stimulation may therefore control the subsequent orientation of cell division so that plant organs and their constituent tissues can develop in an orderly, specified manner. Thus, nuclear movement may be necessary, not only in response to the external environment, but also in response to the developmental microenvironment inside the tissues.
Subcellular Response to Mechanical Stimulations
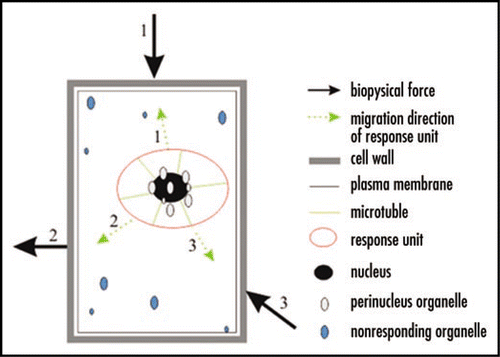
Chloroplasts and other organelles, but not nuclei, have exhibited directional movement in response to mechanical stimulations in lower plants, ferns and bryophytes.Citation14–Citation17 In higher plants, however, non-nuclear organelle movement upon mechanical stimulation has not been reported. We used tobacco ovules that generate bar-shaped cells to detect cytoplasmic changes upon local mechanical stimulation. In this model, some plastids and mitochondria were also observed to participate in the defense response along with the nucleus, creating a response unit that displayed cytoplasmic compartmentalization.Citation12 The subcellular response of an internal cell to repetitive local biophysical forces is shown in .
Interestingly, the phenomenon of nuclear recruitment of the surrounding organelles in response to mechanical stimulation can be understood given the concept of a “cell body” in that the nucleus structurally and functionally organizes perinuclear cytoplasm and can manipulate complexes of the cell periphery.Citation18–Citation21 Given that the ability to respond to mechanical stimulations is fundamental to a single cell, the cytoplasmic compartmental response to mechanical stimulation may reflect the essential nature of the cell. According to the cell body theory,Citation18–Citation21 such functional compartmentalization reflects differences in the origin of the perinuclear cytoplasm and that of peripheral cellular complexes.
Considering the close relationship between nuclear position and the cell division planeCitation13 and the phenomenon of cytoplasmic functional compartmentalization, it is noteworthy to consider equality/inequality during cell division and that cell polarity may be regulated by different mechanisms. Mechanical stimulation is involved in the positioning of the cell division plane, but as yet, there is no evidence for its role in establishing polarity.
Mechanism of Mechanotransduction
Because of the ubiquity of mechanical stimulations, mechanotransduction has been studied for many years, but has only focused on animal cells. Generally, animal cells are considered to be able to convert physical forces into changes in intracellular biochemistry, eventually leading to altered cell morphology. The general underlying molecular pathways have been summarized by Kamm and Kaazempur-Mofrad.Citation22 Force-induced changes in protein structure within cells are hypothesized to expose novel binding sites for ligands.Citation23 Studies in plants are mainly focused on subcellular changes, including the cell wall, cytoplasmic membrane, behavior of the cytoskeleton and organelles, and even division patterns.Citation3,Citation5,Citation8,Citation24 By using plasmolysis and pharmacological methods, Zhou et al.,Citation25 demonstrated that intact microtubules and plasma membrane-cell wall adhesions are required for response to mechanical stimulation in Chrysanthemum cells. Significantly, an essential role of microtubules in regulating cell growth in response to mechanical stimulations has frequently been reported.Citation26,Citation27 The close relationships between the patterns of cell expansion and microtubule orientation have led to the hypothesis that there are biophysical forces generated within tissues and organs that ultimately function to align cortical microtubules and planes of cell division.Citation3,Citation4,Citation26 Our work offers new evidence supporting this hypothesis. However, to further validate the influence of mechanical signals on cell division and nuclear shift via microtubule activity, it is necessary to carefully trace the microtubule dynamics during a cell's reaction to mechanical stimulations. The determination of whether microtubules behave differently in so called “cell body” and “periphery complexes”Citation19–Citation21 will surely facilitate the understanding of both the mechanism of a cell responding to mechanical stimulations and the phenomenon of functional compartmentalization of the cytoplasm.
Figures and Tables
Acknowledgements
The project was supported by National Natural Science Fund of China (90408002, 30771131) and the key grant project of Chinese Ministry of Education (307018).
Addendum to:
References
- Jaffe MJ. Thigmomorphogenisis: the response of plant growth and development to mechanical stimulation. Planta 1973; 114:143 - 157
- Braam J. In touch: plant responses to mechanical stimuli. New Phytol 2005; 165:373 - 389
- Lintilhac PM, Vesecky TB. Stress-induced alignment of division plane in plant tissues grown in vitro. Nature 1984; 307:363 - 364
- Cyr RJ. Microtubules in plant morphogenesis: role of the cortical array. Annu Rev Cell Biol 1994; 10:153 - 180
- Nagai R. Regulation of intracellular movements in plant cells by environmental stimuli. Int Rev Cytol 1993; 145:251 - 310
- Williamson RE. Organelle movement. Ann Rev Plant Physiol Plant Mol Biol 1993; 44:181 - 202
- Kennard JL, Cleary AL. Pre-mitotic nuclear migration in subsidiary mother cells of Tradescantia occurs in G1 of the cell cycle and requires F-actin. Cell Motil Cytoskel 1997; 36:55 - 67
- Gus-Mayer S, Naton B, Hahlbrock K, Schmelzer E. Local mechanical stimulation induces components of the pathogen defense response in parsley. Proc Natl Acad Sci, USA 1998; 95:8398 - 8403
- Xiong TC, Jauneau A, Ranjeva R, Mazars C. Isolated plant nuclei as mechanical and thermal sensors involved in calcium signaling. Plant J 2004; 40:12 - 21
- Gallagher K, Smith LG. Roles for polarity and nuclear determinants in specifying daughter cell fates after an asymmetric division in the maize leaf. Curr Biol 2000; 10:1229 - 1232
- Qu LH, Sun MX. The plant cell nucleus is constantly alert and highly sensitive to repetitive local mechanical stimulations. Plant Cell Rep 2007; 26:1187 - 1193
- Qu LH, Sun MX. Cytoplasmic compartmental response to local mechanical stimulation of internal tissue cells. Protoplasma 2008; 233:51 - 59
- Smith LG. Plant cell division: building walls in the right places. Nat Rev Mol Cell Biol 2001; 2:33 - 39
- Makita N, Shihira-lshikawa I. Chloroplast assemeblage by mechanical stimulation and its intercellular transmission in diatom cells. Protoplasma 1997; 197:86 - 95
- Sato Y, Kadota A, Wada M. Mechanically induced avoidance response of chloroplasts in fern protonemal cells. Plant Physiol 1999; 121:37 - 44
- Sato Y, Wada M, Kadota A. Accumulation response of chloroplasts induced by mechanical stimulation in bryophyte cells. Planta 2003; 216:772 - 777
- Kasahara M, Kagawa T, Oikawa K, Suetsugu N, Miyao M, Wada M. Chloroplast avoidance movement reduces photodamage in plants. Nature 2002; 420:829 - 832
- Mazia D. The cell cycle at the cellular level. Eur J Cell Biol 1993; 61:14
- Baluška F, Barlow PW, Lichtscheidl K, Volkmann D. The plant cell body: a cytoskeletal tool for cellular development and morphogenesis. Protoplasma 1998; 202:1 - 10
- Baluška F, Volkmann D, Barlow PW. Cell bodies in a cage. Nature 2004a; 428:371
- Baluška F, Volkmann D, Barlow PW. Eukaryotic cells and their cell bodies: Cell Theory revised. Ann Bot (Lond) 2004b; 94:9 - 32
- Kamm RD, Kaazempur-Mofrad MR. On the molecular basis for mechanotransduction. Mech Chem Biosyst 2004; 1:201 - 209
- Johnson CP, Tang HY, Carag C, Speicher DW, Discher DE. Forced unfolding of proteins within cells. Science 2007; 317:663 - 666
- Baluška F, Samaj J, Wojtaszek P, Volkmann D, Menzel D. Cytoskeleton-plasma membrane-cell wall continuum in plants. Emerging links revisited. Plant Physiol 2003; 133:482 - 491
- Zhou J, Wang BC, Li Y, Wang YC, Zhu LQ. Responses of Chrysanthemum cells to mechanical stimulation require intact microtubules and plasma membrane-cell wall adhesion. J Plant Growth Regul 2007; 26:55 - 68
- Wymer CL, Wymer SA, Cosgrove DJ, Cyr RJ. Plant cell growth responds to external forces and the response requires intact microtubules. Plant Physiol 1996; 110:425 - 430
- Lynch TM, Lintilhac PM. Mechanical signals in plant development: a new method for single cell studies. Dev Biol 1997; 181:246 - 256