Abstract
Constitutive triple response 1 (CTR1) is a protein kinase that represses plant responses to ethylene. Recently, we have shown that CTR1 function is negatively regulated by the lipid second messenger phosphatidic acid (PA) in vitro. PA was shown to inhibit (1) CTR1’s protein kinase activity, (2) the intramolecular interaction between N-terminus and kinase domain, and (3) the interaction of CTR1 with the ethylene receptor ETR1. PA typically accumulates within minutes in response to biotic or abiotic stresses, which are known to induce ethylene formation. Although long-term treatment with ethephon does stimulate PA accumulation, our results show no fast increase in PA in response to ethylene. A speculative model is presented which explains how stress-induced PA formation could switch on downstream ethylene responses via interaction of the lipid with CTR1.
Role of PA in CTR1 Kinase Regulation
The plant hormone ethylene regulates growth and development and responses to biotic and abiotic stress. By genetic screens, many of the players involved in perception and subsequent signal transduction have been identified.Citation2 In Arabidopsis, ethylene is perceived by a family of receptors, represented by ETR1, ERS1, EIN4, ETR2 and ERS2. In air, these receptors serve to activate constitutive triple response 1 (CTR1), which is a negative regulator of the pathway. CTR1 actively represses EIN2 function, thus inhibiting all downstream ethylene responses.Citation3,Citation4 Upon binding of ethylene, the activity of the receptors is altered and CTR1 is deactivated, which results in activation of ethylene responses. While the genetic evidence is compelling, surprisingly little is known about the molecular mechanism of CTR1 activation. It has been shown that CTR1 has ser/thr kinase activity in vitro and that it can autophosphorylate as well as phosphorylate the artificial substrate myelin basic protein.Citation5 A direct interaction with the ethylene receptors seems to be required for CTR1 function.Citation6
CTR1 is a homolog of the mammalian Raf-1 kinase, a MAP kinase kinase kinase, which is involved in various cellular processes including proliferation and differentiation, both normal and pathological. In order to be activated, Raf-1 must be recruited to the membrane. This recruitment involves binding to the lipid second messenger phosphatidic acid (PA) through a specific lipid-binding site in the protein.Citation7–Citation9 In plants, PA plays a role in many different stress-signaling pathways as well as in normal plant development. Besides being an intermediate in glycerolipid synthesis at the ER, PA is transiently generated via two lipid signaling pathways, involving either phospholipase D (PLD) or phospholipase C, the latter coupled to diacylglycerol kinase activity.Citation10,Citation11
We found that CTR1, like Raf-1, directly interacts with PA.Citation1 The binding site, however, is different than the region identified for Raf-1. In vitro protein kinase activity of CTR1 was shown to be inhibited by PA. Moreover, PA inhibited the intra-molecular interaction of the CTR1 kinase domain with the N-terminus as well as with the interaction with the ethylene receptor ETR1. Together, this biochemical evidence shows inhibition of the action of the negative regulator CTR1 and thus suggests a positive role of PA in ethylene signaling.Citation1
A Model for the Role of Stress-Induced PA in Inducing Ethylene Responses via Inhibition of CTR1
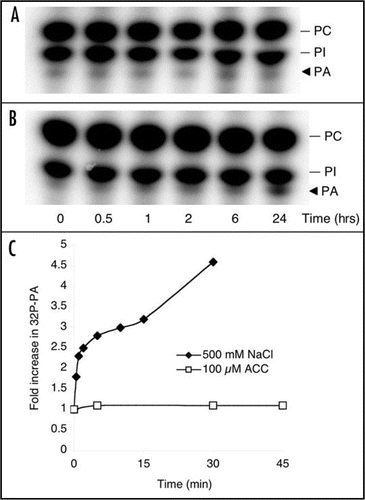
Since PA affected CTR1 function in vitro, it was of interest to determine whether PA is part of the ethylene pathway. Thus, we investigated whether ethylene itself induces a PA response in Arabidopsis seedling and suspension-cultured cells. However, no rapid PA accumulation was observed in either cells or seedlings in response to ethephon or ACC (). As a positive control, cells were treated with NaCl, which induced accumulation of PA within minutes (). As PA formation does not seem to occur as a direct response to ethylene perception, we propose the role of PA in ethylene signaling to be independent of ethylene recognition. Several stress stimuli, such as wounding, pathogenic elicitors and salt do give a rapid PA response and are known to trigger ethylene responses.Citation11–Citation15 It is possible that this increase in PA leads to inhibition of CTR1, which in turn would relieve repression of the EIN2 pathway and induce downstream ethylene signaling.
This hypothesis is the basis for our working model on the role of PA in ethylene signaling, which is presented in . In the absence of ethylene, the ethylene receptors bind and activate the CTR1 kinase, resulting in suppression of downstream ethylene signaling. In the presence of ethylene, CTR1 dissociates from the receptors and becomes inactive, resulting in derepression of the downstream ethylene pathwayCitation3 (). builds further on the existing model and suggests an alternative or complementary pathway to inhibit CTR1 kinase activity and to induce ethylene responses in the absence of ethylene but in the presence of stress-induced PA. The inhibition of CTR1 kinase by PA could be a fast way to switch on the ethylene pathway in response to stress, without the need to generate ethylene first. Alternatively, PA dependent inhibition of CTR1 could function synergistically with ethylene and work to promote rapid and/or heightened activation of ethylene responses. This mechanism could act in parallel with other recently described stress-activated pathways that induce ethylene production through induction of ACC synthase (ACS) gene expressionCitation16 and ACS activity.Citation12 An advantage of the PA pathway would be its speed.
Long-term Ethylene Mediated Responses
Since ethephon treatment did give a PA response after 24 hours (; also observed before by Lee et al.Citation17), this may be significant for the progression or maintenance of long-term ethylene-regulated responses. Many ethylene-mediated phenomena, including autocatalytic ethylene regulated senescence, e.g., fruit ripening and flower senescence, are long-term phenomena. PA could represent a secondary mechanism to ensure inactivation of CTR1 and the progression of developmental processes. In support of PA's role, silencing of PLDα1, one of the enzymes that can produce PA, has been shown to delay ethylene-induced senescence.Citation18
Conclusions
Evidence is provided for a novel regulator of CTR1 activity; the signaling lipid PA. Its proposed function in vivo is fully consistent with both ethylene and PA signaling and their relation to stress. Our future work will focus on elucidating the implications of PA-binding for CTR1 function and ethylene responses.
Figures and Tables
Figure 1 Only long-term ethylene treatment triggers PA accumulation in Arabidopsis suspension cells. Arabidopsis seedlings (A and C) or suspension cells (B) were labeled with 32Pi and subsequently treated with 500 µM ethephon (A and B) or 100 µM ACC (C). As a positive control for PA accumulation, seedlings were treated with 500 mM NaCl (C). Phospholipids were extracted, separated by alkaline TLC, visualized by autoradiography and quantified by PhosphoImaging.
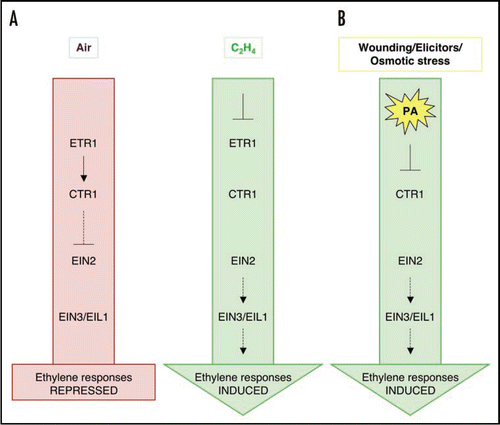
Figure 2 Model showing how PA could induce ethylene responses by inhibition of CTR1 activity and its interaction with the ethylene receptor ETR1. (A) Current model (adapted from Chen et al.Citation3) on the ethylene signaling cascade in air and in the presence of ethylene. Note that CTR1 is a negative regulator of all downstream ethylene responses. (B) By directly interfering with CTR1 activity, PA could in theory induce downstream ethylene responses even in the absence of ethylene itself. Alternatively, PA could supplement the effects of ethylene on CTR1 activity, potentially resulting in an increase in level of response with regard to magnitude or length.
Addendum to:
References
- Testerink C, Larsen PB, van der Does D, van Himbergen JAJ, Munnik T. Phosphatidic acid binds to and inhibits the activity of Arabidopsis CTR1. J Exp Bot 2007; 58:3905 - 3914
- Guo H, Ecker JR. The ethylene signaling pathway: new insights. Curr Opin Plant Biol 2004; 7:40 - 49
- Chen YF, Etheridge N, Schaller GE. Ethylene signal transduction. Ann Bot 2005; 95:901 - 915
- Benavente LM, Alonso JM. Molecular mechanisms of ethylene signaling in Arabidopsis. Mol Biosyst 2006; 2:165 - 173
- Huang Y, Li H, Hutchison CE, Laskey J, Kieber JJ. Biochemical and functional analysis of CTR1, a protein kinase that negatively regulates ethylene signaling in Arabidopsis. Plant J 2003; 33:221 - 233
- Gao Z, Chen YF, Randlett MD, Zhao XC, Findell JL, Kieber JJ, Schaller GE. Localization of the Raf-like kinase CTR1 to the endoplasmic reticulum of Arabidopsis through participation in ethylene receptor signaling complexes. J Biol Chem 2003; 278:34725 - 34732
- Ghosh S, Strum JC, Sciorra VA, Daniel L, Bell RM. Raf-1 kinase possesses distinct binding domains for phosphatidylserine and phosphatidic acid. Phosphatidic acid regulates the translocation of Raf-1 in 12-O-tetradecanoylphorbol-13-acetate-stimulated Madin-Darby canine kidney cells. J Biol Chem 1996; 271:8472 - 8480
- Ghosh S, Moore S, Bell RM, Dush M. Functional analysis of a phosphatidic acid binding domain in human Raf-1 kinase: mutations in the phosphatidate binding domain lead to tail and trunk abnormalities in developing zebrafish embryos. J Biol Chem 2003; 278:45690 - 45696
- Rizzo MA, Shome K, Vasudevan C, Stolz DB, Sung TC, Frohman MA, Watkins SC, Romero G. Phospholipase D and its product, phosphatidic acid, mediate agonist-dependent raf-1 translocation to the plasma membrane and the activation of the mitogen-activated protein kinase pathway. J Biol Chem 1999; 274:1131 - 1139
- Wang X, Devaiah SP, Zhang W, Welti R. Signaling functions of phosphatidic acid. Prog Lipid Res 2006; 45:250 - 278
- Testerink C, Munnik T. Phosphatidic acid: a multifunctional stress signaling lipid in plants. Trends Plant Sci 2005; 10:368 - 375
- Liu Y, Zhang S. Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell 2004; 16:3386 - 3399
- Felix G, Grosskopf DG, Regenass M, Basse CW, Boller T. Elicitor-induced ethylene biosynthesis in tomato cells—characterization and use as a bioassay for elicitor action. Plant Physiol 1991; 97:19 - 25
- Felix G, Regenass M, Boller T. Sensing of osmotic pressure changes in tomato cells. Plant Physiol 2000; 124:1169 - 1180
- O'Donnell PJ, Calvert C, Atzorn R, Wasternack C, Leyser HMO, Bowles DJ. Ethylene as a signal mediating the wound response of tomato plants. Science 1996; 274:1914 - 1917
- Matarasso N, Schuster S, Avni A. A novel plant cysteine protease has a dual function as a regulator of 1-aminocyclopropane-1-carboxylic Acid synthase gene expression. Plant Cell 2005; 17:1205 - 1216
- Lee SH, Chae HS, Lee TK, Kim SH, Shin SH, Cho BH, Cho SH, Kang BG, Lee WS. Ethylene-mediated phospholipid catabolic pathway in glucose-starved carrot suspension cells. Plant Physiol 1998; 116:223 - 229
- Fan L, Zheng S, Wang X. Antisense suppression of phospholipase Dα retards abscisic acid- and ethylene-promoted senescence of postharvest Arabidopsis leaves. Plant Cell 1997; 9:2183 - 2196