Abstract
Endosperm is an interesting model for in vitro experiments, because of its unique origin, development and ploidy level. Here we used Actinidia deliciosa endosperm-derived callus to investigate morphology, histology and chemistry of extracellular matrix (ECM) structures in morphogenically stable tissue from long - term culture. SEM and TEM analysis showed that ECM is a heterogenous layer which consists of amorphous, dark – staining material, osmiophilic granules and reticulated fibres outside the outer callus cell wall. This structure may serve as a structural marker of morphogenic competence in endosperm – derived callus, because of its presence on the surface of callus forming morphogenic domains and its disappearance during organ growth. Based on immunolabelling, histochemistry, solvent and enzyme treatments, we suggest that pectins and lipids are components of the ECM layer. These results might indicate protective, water retention and/or cell communication functions for this ECM layer.
Addendum to: Popielarska-Konieczna M, Kozieradzka-Kiszkurno M, Świerczyńska J, Góralski G, Ślesak H, Bohdanowicz J. Ultrastructure and histochemical analysis of extracellular matrix surface network in kiwifruit endosperm-derived callus culture. Plant Cell Rep 2008; 27:1137-45.
In several plants cultured in vitro, SEM analysis revealed that induction of morphogenesis is linked to the appearance of a fibrillar network referred to as the extracellular matrix surface network (ECMSN).Citation1 Šamaj et al.,Citation2,Citation3 reported, that the ECMSN plays an important morphoregulatory role during somatic embryogenesis and organogenesis, implying an active role in plant morphogenesis. According to Bobák et al.,Citation4 the chemical composition and structural arrangement of the ECMSN on the cell surface indicate that it may play a fundamental role in cell-to-cell recognition and interaction, cell division and differentiation, and also in generation and maintenance of some traits in plant cell populations. There is not a large body of data confirming the occurrence of ECM during in vitro organogenesis.Citation5,Citation6 Most data concern the formation, structure and chemical composition and function of ECM in somatic and androgenic embryogenesis (reviewed in refs. Citation6–Citation10). In previous histological and SEM analysis of morphogenic endosperm-derived callus of A. deliciosa, we described the presence of a membranous layer covering the callus surface, which we termed extracellular matrix (ECM).Citation11 The present experiments employed SEM, TEM and histochemical analysis to study the structure and composition of material coating the callus surface.
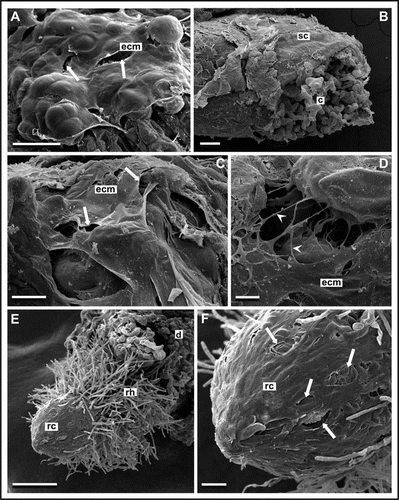
SEM revealed the presence of heterogeneous material covering the callus surface. A smooth membranous layer coated some parts of the callus surface (), while other regions were covered by fibrillar structures and granules of mucilage-like secretion forming a network at site of cell-cell adhesion. Similar structures were observed during in vitro induction of somatic embryogenesis, androgenesis and organogenesis in different species (reviewed in refs. Citation7–Citation13).
TEM showed a layer composed of amorphous material with fibrillar and spherical components covering morphogenic cell clusters. The cell wall of non-embryogenic and senescent cells was also covered with ECM, but it differed in appearance: the layer was granular, with amorphous deposits. According to Rumyansteva et al.,Citation14 loss of embryogenic competence was preceded by modification of the ECM structure from fibrillar to glue-like.
Callus induction was observed after 2–3 weeks of culture (). Membraneous and fibrillar structures were visible on the very small callus surface by SEM ( and D), though the first signs of organogenesis were noted 4–6 weeks later. TEM observations of the first steps of callogenesis also showed fine, amorphous ECM on the cell surface (data not shown). This might suggest that the extracellular matrix described above probably has a protective rather than signalling function.
SEM of root tips appearing during culture showed that root cap cells were enveloped by a specific, partially damaged layer ( and F) similar in appearance to membraneous ECM on the callus surface. It is commonly known, that terminal part of the root tip is composed of pectins and performs a protective function, but signalling molecules were also detected on the root tip. In maize roots in vivo, Abeysekera and McCullyCitation15 found ECM occurring as a pellicle covering young meristematic epidermal cells, which contained arabinogalactan proteins.
Solvent treatment and histochemical staining with Sudan Black B suggested that some lipophilic substances are part of the ECM in kiwifruit. Damage to the membranous layer observed by SEM after solvent treatment might be the results of removal of lipid components from the ECM. Moreover, TEM of the surface layer revealed globular deposits similar in electron density and appearance to lipid bodies. Apart from Actinidia, lipophilic components of the ECM have been reported only in Triticum.Citation10 The probable presence of lipids in the examined ECM may be connected with protective and signalling function, especially in oxygen stress conditions.Citation16 Pectinase digestion caused only partial disappearance of the ECM, exposing granular remnants on the cell surface and filaments forming thick fibrils at sites of cell adhesion, visible by SEM. Cellulose-binding fluorescent dye ruled out cellulose polymers as an element of ECM structure. Additionally, we identified a callose component in morphogenic callus. Deposition of polysaccharides around embryogenic cells has been suggested by Šamaj et al.,Citation3 as an early structural marker for these cells.
During these experiments we detected JIM5-labelled low-methylesterified pectins in intercellular spaces and on the surface layer. A comparison of our results with those obtained in TriticumCitation6 and ZeaCitation3 supports Šamaj et al.,Citation3 assumption that regulation of pectin localization within the ECM differs between monocotyledonous and dicotyledonous.
Our results suggest the possible role of the extracellular material during kiwifruit regeneration. Pectins serve as a reservoir of signalling molecules and for water retention. Consequently, ECM may be involved in integration and recognition of morphogenic cells within multicellular callus domains, and a protective function cannot be excluded.
Abbreviations
| ECM | = | extracellular matrix |
| ECMSN | = | extracellular matrix surface network |
| SEM | = | scanning electron microscopy |
| TEM | = | transmission electron microscopy |
Figures and Tables
Figure 1 SEM images of kiwifruit endosperm-derived callus after callogenesis induction, long-term culture and organogenesis. (A) Layer composed of membranous, partially damaged (arrows) structure enveloping callus surface after long-term culture. (B–D) Callus induction after 3 weeks of culture. Remnants of seed coat (sc) and cells of non-morphogenic callus (c) are observed (B). However, some part of the callus surface is covered with fibrillar (arrowheads) and membranous extracellular matrix (ecm) with holes (arrows) (C and D). (E and F) Rhizogenesis after 6 weeks of culture. Root with root cap (rc) and root hairs (rh) is emerging from callus domain (d). Partly damaged (arrows) membranous material covering cells of root tip (F). Bars represent 50 µm for (D); 100 µm for (C and F); 200 µm for (A and B); and 500 µm for (E).
Addendum to:
References
- Šamaj J, Bobák M, Blehová A, Krištin J, Auxtová-Šamajová O. Developmental SEM observations on an extracellular matrix in embryogenic calli of Drosera rotundifolia and Zea mays. Protoplasma 1995; 186:45 - 49
- Šamaj J, Baluška F, Bobák M, Volkmann D. Extracellular matrix surface network of embryogenic units of friable maize callus contains arabinogalactan-proteins recognized by monoclonal antibody JIM4. Plant Cell Rep 1999; 18:369 - 374
- Šamaj J, Bobák M, Blehová A, Pret'ová A. Mujib A, Šamaj J. Importance of cytoskeleton and cell wall in somatic embryogenesis. Somatic embryogenesis 2006; Berlin Heidelberg Springer-Verlag
- Bobák M, Šamaj J, Hlinkova E, Hlavacka A, Ovecka M. Extracellular matrix in early stages of direct somatic embryogenesis of Drosera spathulata. Biol Plant 2003/4; 47:161 - 162
- Ovecka M, Bobák M. Structural diversity of Papaver somniferum L. cell surfaces in vitro depending on particular steps of plant regeneration and morphogenetic program. Acta Physiol Plant 1999; 21:117 - 126
- Konieczny R, Swierczynska J, Czaplicki AZ, Bohdanowicz J. Distribution of pectin and arabinogalactan protein epitopes during organogenesis from androgenic callus of wheat. Plant Cell Rep 2007; 26:355 - 363
- Chapman A, Helleboid S, Blervacq AS, Vasseur J, Hilbert JL. Removal of the fibrillar network surrounding Cichorium somatic embryos using cytoskeletal inhibitors: analysis of proteic components. Plant Sci 2000a; 150:103 - 114
- Chapman A, Blervacq AS, Tissier JP, Denbreil B, Vasseur J, Hilbert JL. Cell wall differentiation during early somatic embryogenesis in plants I. Scanning and transmission electron microscopy originating from direct, indirect and adventitious pathways. Can J Bot 2000b; 78:816 - 823
- Namasivayam P, Skepper J, Hanke D. Identification of a potential structural marker for embryogenic competency in the Brassica napus spp. oleifera embryogenic tissue. Plant Cell Rep 2006; 25:887 - 895
- Konieczny R, Bohdanowicz J, Czaplicki AZ, Przywara L. Extracellular matrix surface network during plant regeneration in wheat anther culture. Plant Cell Tiss Organ Cult 2005; 83:201 - 208
- Popielarska M, Slesak H, Góralski G. Histological and SEM studies on organogenesis in endosperm-derived callus of kiwifruit (Actinidia deliciosa cv. Hayward). Acta Biol Cracov Ser Bot 2006; 48:97 - 104
- Verdeil JL, Hocher V, Huet C, Grosdemange F, Escoute J, Ferrière N, Nicole M. Ultrastructural changes in coconut calluses associated with the acquisition of embryogenic competence. Ann Bot 2001; 88:9 - 18
- Dubois T, Guedira M, Dubois J, Vasseur J. Direct somatic embryogenesis in leaves of Cichorium. A histological and SEM study of early stages. Protoplasma 1991; 162:120 - 127
- Rumyansteva NI, Šamaj J, Ensikat HJ, Sal'nikov VV, Kostyukova YA, Baluška F, Volkmann D. Changes in the extracellular matrix surface network during cyclic reproduction of proembryogenic cell complex in the Fagopyrum tataricum (L.) Gaertn callus. Dokl Biol Sci 2003; 391:375 - 378
- Abeysekera RM, McCully ME. The epidermal surface of the maize root tip I. Development in normal roots. New Phytologist 1993; 125:413 - 429
- Mueller MJ. Archetype signals in plants: the phytoprostanes. Curr Opin Plant Biol 2004; 7:441 - 448