Abstract
It has now been demonstrated that treatment of Arabidopsis thaliana plants with glycine betaine (GB) improves tolerance to chilling stress by regulating gene expression. This finding provides the opportunity to identify new stress determinants using gene expression profiling with microarrays followed by functional confirmation of the involvement of candidate genes via mutant studies. The first gene identified by this approach was the gene for RabA4c GTPase (At5g47960), which is expressed in roots and is involved in vesicle trafficking from the Golgi Apparatus to the plasma membrane. Recently, we have identified the FRO2 ferric reductase (At1g01580) which is localized on the plasma membrane, as another component of the GB-regulated system and suggested that enhanced production of reductant in the cell wall also plays a role in chilling tolerance. This addendum article focuses on the concept that extracellular processes may play a pivotal role in stress tolerance. A candidate gene list is presented for GB-upregulated genes in Arabidopsis roots and a model is proposed incorporating candidate genes with potential roles in relation to reactive oxygen species (ROS) signaling and chilling stress.
Development of Arabidopsis as a model system has led to new experimental approaches for obtaining a better understanding of (cold) stress tolerance by (1) large-scale screening for mutants that are less tolerantCitation1 or more tolerantCitation2 to cold temperatures; (2) gene expression studies to identify genes affected by cold temperaturesCitation3,Citation4 and (3) chemical genetic approaches based on ligands such as glycine betaine (GB) that regulate the expression of genes playing a role in stress tolerance.Citation5 Work by N. Murata and colleaguesCitation6 during the last 10 years has been pivotal in demonstrating that GB can confer tolerance to several types of stress, including stresses caused by chilling, frost, salt, drought and high light intensities either after application to plants or in transgenics engineered to overproduce GB. Nevertheless, when we started our investigations a few years ago, it was unclear whether GB's effect could be attributed to gene expression changes.
The first step in testing the idea that gene expression changes are involved in GB-mediated stress tolerance was to conduct gene expression profiling using microarrays. shows a candidate gene list developed for Arabidopsis roots based on significant increases in gene expression resulting from GB treatment. The most interesting aspects of this list are the prevalence of genes encoding reactive oxygen species (ROS) scavenging enzymes, targeted either intra- and extracellularly, as well as genes encoding functions related to membrane trafficking (RabA4c GTPase) and to extracellular ferric reduction (FRO2 and FRO4). Given the fact that GB can protect plants from several types of stress, including stresses caused by chilling, frost, salt, drought and high light intensities, it is interesting to ask whether the response to each of these stress types involves ROS signaling. If this is so, stress-induced ROS signaling processes may be interesting targets for cultivar improvement.
To prove that gene expression was required for GB's effect, we set out to obtain functional evidence for a direct role of candidate genes. In the case of the FRO2 ferric reductase gene, this evidence was provided using the FRO2-null mutant frd1-1. Although Arabidopsis is usually defined as chilling-resistant because it shows no obvious signs of chilling injury, chilling does have an effect in this species because chilled plants show inhibited root growth upon transfer back to normal temperatures. Remarkably, when wild type plants were pretreated with GB, root growth rates after chilling were comparable to non-chilled plants. GB also prevented ROS accumulation during chilling. In contrast to wild type, the frd1-1 mutant showed no GB response in the chilling test, either in terms of root growth rates or ROS accumulation, proving the requirement for an active FRO2 gene for GB's effect in the chilling response. Using the wild type, we took the research one step further,Citation7 showing that GB pretreatments actually resulted in increases in ferric reductase enzyme activities during chilling, thus providing direct biochemical evidence that a mechanism for transferring reductant from the cytoplasm to the cell wall is activated by GB.
With the recognition that ROS signaling is tightly coupled to cold stress, the mechanism of ROS production in the cold becomes of special interest. In this regard, plasma membrane (PM) NADPH oxidase activity (NOX) is an interesting possibilityCitation8 inasmuch as NOX has been demonstrated to be involved in ROS production during root hair development by root epidermal cells as well as being implicated in pathogen responses and stomatal control. By analogy to animal systems, the activation of NOX in phagocytes has been studied extensively, revealing several required cofactors in addition to phosphorylation events and membrane trafficking.Citation9 It is also known that phagocyte NOX produces superoxide on the outside of the plasma membrane which would correspond to the wall region in plant cells. Thus, according to our model in , chilling activates NOX, leading to superoxide production and ROS signaling associated with chilling stress.Citation10 In the presence of GB, ROS accumuation is overridden by GB-mediated upregulation of ascorbate oxidase and monodehydroascorbate reductase (MDHAR) along with upregulation of RabA4c GTPase (shown bound to a PM tethering proteinCitation11) which enhances export of these enzymes to the cell wall. Another critical component of the model is the upregulation of PM FRO2 ferric reductase which uses cytosolic NADPH to reduce ferric ions in the cell wall, generating reductant potential to fuel the breakdown of hydrogen peroxide to oxygen and water. An interesting unanswered question is how ferrous reductant potential (designated as [H] in the ) is transferred to hydrogen peroxide via ascorbate. Presumably, the reactions involved must be highly efficient so that ferrous ion levels are maintained at low levels. Otherwise, ferrous ions could react directly with hydrogen peroxide by the Fenton reaction,Citation8 generating highly reactive ROS species such as the hydroxyl radical.
The demonstration that FRO2 plays a role in GB-mediated chilling tolerance and ROS accumulation represents a new physiological function for FRO genes, in addition to their demonstrated role in iron uptake.Citation12,Citation13 Rather than focusing only on iron uptake aspects, our findings suggest that FRO genes might play a pivotal role in balancing the ROS status of whole cells and organelles in relation to ROS signalng processes.
Figures and Tables
Figure 1 Model for how GB-upregulated genes in roots of Arabidopsis could prevent ROS buildup in cell walls, preventing ROS signaling associated with chilling stress. Abbreviations used in the Figure are described in the text. SOD is superoxide dismutase.
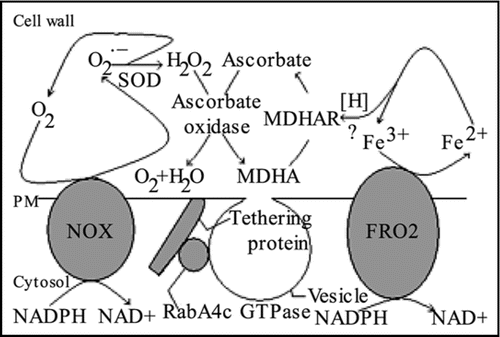
Table 1 List of GB-candidate genes in Arabidopsis roots based on gene expression profiling with microarrays
Addendum to:
References
- Thorlby G, Fourrier N, Warren G. The SENSITIVE TO FREEZING2 gene, required for freezing tolerance in Arabidopsis thaliana, encodes a β-glucosidase. Plant Cell 2004; 16:2192 - 2203
- Xin Z, Browse J. eskimo1 mutants are constitutively freezing-tolerant. Proc Natl Acad Sci USA 1998; 95:7799 - 7804
- Stockinger EJ, Gilmour SJ, Thomashow MF. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcription activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci USA 1997; 94:1035 - 1040
- Viswanathan C, Zhu J-K. Molecular genetic analysis of cold-regulated gene transcription. Phil Trans Royal Soc London B 2002; 357:877 - 886
- Einset J, Nielsen E, Connolly EL, Bones A, Sparstad T, Winge P, et al. Membrane trafficking RabA4c involved in the effect of glycine betaine on recovery from chilling stress in Arabidopsis. Physiol Plant 2007; 130:511 - 518
- Chen THH, Murata N. Glycinebetaine: an effective protectant against abiotic stress in plants. Trends Plant Sci 2008; 13:499 - 505
- Einset J, Winge P, Bones A, Connolly EL. The FRO2 ferric reductase is required for glycine betaine's effect on chilling tolerance in Arabidopsis roots. Physiol Plant 2008; 134:334 - 341
- Foyer CH, Noctor G. Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environm 2005; 28:1056 - 1071
- Bedar K, Krause K-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 2007; 87:245 - 313
- Einset J, Winge P, Bones A. ROS signaling pathways in chilling stress. Plant Signal Behav 2007; 2:365 - 367
- Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci USA 2006; 103:11821 - 11827
- Jeong J, Cohu C, Kerkeb L, Pilon M, Connolly EL, Guerinot ML. Chloroplast Fe(III) chelate reductase activity is essential for seedling viability under iron limiting conditions. Proc Natl Acad Sci USA 2008; 105:10619 - 10624
- Robinson NJ, Procter CM, Connolly EL, Guerinot ML. A ferric-chelate reductase for iron uptake from soils. Nature 1999; 397:694 - 697
- Emanuelsson O, Nielsen H, Brunak S, von Heijne G. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol 2000; 300:1005 - 1016
- Reumann S, Babujee L, Ma C, Wienkoop S, Siemsen T, Antonicelli E, et al. Proteome analysis of Arabidopsis leaf peroxisomes reveals novel targeting peptides, metabolic pathways and defense mechanisms. Plant Cell 2007; 19:3170 - 3193