Abstract
Quality control of proteins in eukaryotic organelles is predominantly maintained by members of the ATP-dependent proteases. Even though numerous biological analyses have shed light on the functional implications of such proteases, their involvement in developmental processes of multicellular organisms has not been determined. We recently identified two lon1 mutant alleles, both missing the carboxy terminal proteolytic domain, that show post-embryonic growth retardation resulting in delayed seedling establishment. In this addendum, we enlighten the role of Lon1 selective proteolysis in plant mitochondria biogenesis, a prerequisite for post-embryonic development and growth. In contrast to the weak lon1-2 allele, the polypeptide encoded by the strong lon1-1 allele carries the sensor- and substrate-discrimination domain allowing substrate recognition and binding. This type of molecular recognition hinders further degradation by the complementary Lon-independent proteolytic machineries resulting in an extra deleterious accumulation of protein aggregates into lon1-1 mitochondria. The most challenging and informative task will be to identify the recognition motifs on the Lon protein substrates and elucidate the molecular events that control plant mitochondrial differentiation.
Maintenance of biological functions requires the constant cycling between protein synthesis and degradation. Selective proteolysis occurs in various cellular compartments to remove short-lived regulatory proteins and to prevent the potentially harmful accumulation of non-native polypeptides. The ubiquitin/26S proteasome pathway has evolved as the main highly selective proteolytic mechanism in the cytoplasm and nucleus modulating several aspects of plant development.Citation1,Citation2 In plant organelles however, protein quality control is performed by ATP-dependent proteases that belong to the Clp, FtsH and Lon families.Citation3–Citation6 These proteases are members of the AAA+ protein superfamily (ATPases associated with diverse cellular activities).Citation7,Citation8
Arabidopsis has four genes encoding for Lon proteases that contain a Serine (S)-Lysine (K) catalytic dyadCitation4 and, like their bacterial and eukaryotic homologs, may combine proteolytic and chaperone-like activities.Citation5,Citation6 Presumably oxidative stress induces Lon-like proteolysis.Citation9,Citation10 Nevertheless, the only designated function of Lon proteases to date is the control of cytoplasmic male sterility in common bean plants.Citation11 We recently characterized two mutant alleles of Arabidopsis Lon1 gene, lon1-1 caused by EMS mutagenesis resulting in a premature termination codon and lon1-2 mutant allele caused by T-DNA insertion.Citation12 Both mutations occur at the 18th exon of AtLon1 gene, which consists of 19 exons. The main phenotypic feature of lon1 mutants is the post-embryonic growth retardation resulting in delayed seedling establishment. This growth retardation remains throughout the entire life cycle of the plant. Biometrics revealed that lon1-2 allele corresponded to a weak mutant allele in comparison to the strong lon1-1 mutant phenotype ().
We postulate that ATP-dependent Lon1 protease is essential for controlling the quality of newly imported or synthesized mitochondrial polypeptides, comprising an essential regulatory mechanism towards mitochondria biogenesis. However, the possibility that Lon1 could function as a protease or chaperone on pre-existing protein moieties within undifferentiated mitochondria is not excluded. Approximately 95% of the mitochondrial proteome is encoded from the nucleus.Citation13 Hence, most of the polypeptides are imported into the organelle. Components of the mitochondrial protein import apparatus are highly abundant in promitochondrial structures and operational during the onset of dry seed embryos imbibition.Citation14 Nevertheless, these components are barely detected in mature mitochondria.Citation14–Citation16 Moreover, the promitochondria proteome is significantly different from that identified in metabolic capable mature mitochondria.Citation14 The degradation of the mitochondrial import machinery components appears to occur with the shift in the mitochondrial population from promitochondria, to mature mitochondria. Increases in oxygen consumption and respiration are observed during this heterotrophic growth period. The activity of mitochondrial proteases may modulate the developmental transition between the two mitochondria types, through the quality control of the imported or newly synthesized polypeptides and the selective degradation of pre-existing proteins. Several lines of experimental evidence support this hypothesis. AtLon1 is targeted to plant mitochondria and complements the respiratory-deficient phenotype of the yeast PIM1 gene homolog. Yeast pim1 mutant mitochondria have irregular shape and accumulate protein aggregates in the matrix.Citation17 The activities of respiratory chain complexes and TCA cycle enzymes are decreased in lon1 mutants, indicating malfunction of these pathways. Transmission electron micrographs indicated that lon1-1 mitochondria morphology is reminiscent to maizeCitation18 and riceCitation14 promitochondria from dry seed embryos. The germination efficiency of lon1 mutants is dramatically diminished under heat-shock conditions (32°C), indicating the existence of a signaling mechanism that orchestrates mitochondria differentiation and seed germination. Ultimately, the mobilization of storage lipids for germination requires not only the peroxisomal biochemical pathways, such as β-oxidation and the glyoxylate cycle, but also specific enzymatic reactions performed in mature plant mitochondria. Mutations that compromise storage lipid mobilization result in post-embryonic growth retardation leading to impaired seedling establishment, as in the case of lon1 mutants.Citation19,Citation20
We further propose a mechanism for the molecular recognition of protein substrates by Lon1 protease. The structural core of Lon1 protease is the AAA+ module (residues 484–810) that consists of two fundamental domains: the nucleotide-binding (α/β) domain and the helical (α) domain. The α/β-domain contains the conserved motifs sensor-1, Walker A and B. The α-domain contains the sensor-2 motif that is characterized by a conserved Arginine residue. The AAA+ module is involved in ATP hydrolysis and in protein substrate remodeling.Citation7,Citation8 Adjacent to the AAA+ module is the sensor- and substrate-discrimination (SSD) domain (). This domain is mainly involved in modulating selective substrate recognition by Lon proteases so as the target protein to be either degraded or properly folded.Citation21 In line with its highly selective mode of action, the SSD domain is substantially diverse between Lon proteases (; ). The strong lon1-1 mutant allele contains the entire SSD domain, while the weak lon1-2 allele carries only a minor segment ( and B). The truncated polypeptide encoded by lon1-1 mutant allele potentially participates in protein substrate recognition and binding due to the activity of the SSD domain. Consequently, while it recognizes the substrate proteins, no further degradation is accomplished due to the absence of the proteolytic (P) domain (residues 811–985). This type of protein recognition most likely leads to delayed degradation of the misfolded or non-native polypeptides by the complementary Lon-independent proteolytic machineries, which are also abundant in the mitochondria matrix. This may result in the formation of high-molecular weight aggregates deleterious for mitochondria organization and function, causing the severe lon1-1 growth phenotype. Experimental observations in yeast revealed the overlapping substrate specificity between the m-AAA high-molecular weight complex of mitochondrial FtsH proteases and the yeast Lon protease homolog PIM1.Citation22,Citation23 Furthermore, the SSD domains of the bacterial Clp ATPases and Lon protease that interact with substrates have similar predicted structures.Citation21 The lon1-2 allele lacks the SSD domain being unable to recognize target polypeptides and, plausibly does not lead to the formation of high-molecular weight aggregates. Nevertheless, the non-native polypeptides still accumulate resulting in the aberrant but rather weak lon1-2 phenotype.
Elevated temperatures increase the fraction of high-molecular weight aggregates within cellular compartments. Under these conditions lon1-1 germination efficiency was dramatically diminished, whereas lon1-2 seeds exhibited relatively better germination.Citation12 Thus, loss of Lon1 proteolysis sustains the conditional formation of supramolecular protein structures with prolonged deleterious effects on seed germination indicating that mitochondria differentiation substantiates seedling establishment and development. The marginal nevertheless noticeable difference in germination rate between the lon1 alleles further confirms that the Lon1-1 polypeptide forms auxiliary aggregates, due to the activity of the SSD domain.
In conclusion, our study has revealed the critical role for AtLon1 protease in organelle biogenesis and seedling establishment. The hypothesis we propose here should provide novel insights into the developmental mechanisms of selective proteolysis in plant mitochondria. Further analysis using a combinatorial approach, mutant analysis and proteomics, is required to decipher the molecular recognition of protein substrates by Lon1 protease and the events that control plant mitochondrial differentiation.
Figures and Tables
Figure 1 The growth phenotype of lon1 mutants. Growth retardation of lon1-2 plants is milder than the lon1-1 mutant allele. Bar: 1 cm.

Figure 2 The structural features of AtLon1 protease and the locations of the identified mutations. (A) Schematic representation of AtLon1 protease outlining the domain structures with important consensus sequences in lon1 mutant alleles. Numbers in parentheses represent amino acid residues defining the conserved domains. (B) Multiple sequence alignment reveals that the SSD is the most variable domain between evolutionary diverse Lon accessions. Box encompasses the amino acid residues of sensor-2 motif. In contrast to the strong lon1-1 allele (asterisk), the weak lon1-2 mutant allele (inverted triangle) bears part of the SSD domain.
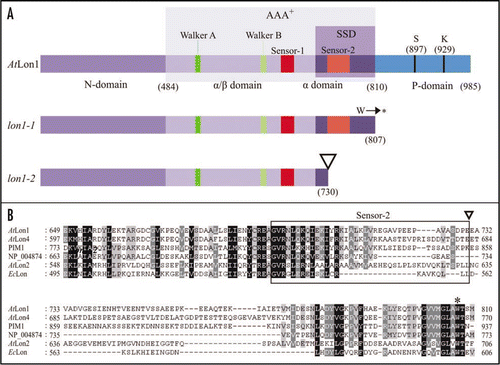
Table 1 Percentages of amino acid identity and similarity of the structural domains between AtLon1Table Footnotea and the arabidopsis AtLon2 and AtLon4 or the non-plant Lon homologsTable Footnoteb
Acknowledgements
This work was supported by Pythagoras grants I and II from Greece to S.R. and P.H. and by the EU ERA-Plant Genomics programme to L.J.S. G.D. is indebted to the Greek SSF.
Addendum to:
References
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem 1998; 67:425 - 479
- Smalle J, Vierstra RD. The ubiquitin 26S proteasome proteolytic pathway. Annu Rev Plant Physiol Plant Mol Biol 2004; 55:555 - 590
- Adam Z, Adamska I, Nakabayashi K, Ostersetzer O, Haussuhl K, Manuell A, et al. Chloroplast and mitochondrial proteases in Arabidopsis. A proposed nomenclature. Plant Physiol 2001; 125:1912 - 1918
- Sinvany-Villalobo G, Davydov O, Ben-Ari G, Zaltsman A, Raskind A, Adam Z. Expression in multigene families. Analysis of chloroplast and mitochondrial proteases. Plant Physiol 2004; 135:1336 - 1345
- Janska H. ATP-dependent proteases in plant mitochondria: What do we know about them today?. Physiol Plant 2005; 123:399 - 405
- Sakamoto W. Protein degradation machineries in plastids. Annu Rev Plant Biol 2006; 57:599 - 621
- Neuwald AF, Aravind L, Spouge JL, Koonin EV. AAA+: A class of chaperone-like ATPases associated with the assembly, operation and disassembly of protein complexes. Genome Res 1999; 9:27 - 43
- Iyer LM, Leipe DD, Koonin EV, Aravind L. Evolutionary history and higher order classification of AAA+ ATPases. J Struct Biol 2004; 146:11 - 31
- Sweetlove LJ, Heazlewood JL, Herald V, Holtzapffel RH, Day DA, Leaver CJ, et al. The impact of oxidative stress on Arabidopsis mitochondria. Plant J 2002; 32:891 - 904
- Lister R, Chew O, Lee MN, Heazlewood JL, Clifton R, Parker KL, et al. A transcriptomic and proteomic characterization of Arabidopsis mitochondrial protein import apparatus and its response to mitochondrial dysfunction. Plant Physiol 2004; 134:777 - 789
- Sarria R, Lyznik A, Vallejos CE, Mackenzie SA. A cytoplasmic male sterility-associated mitochondrial peptide in common bean is post-translationally regulated. Plant Cell 1998; 10:1217 - 1228
- Rigas S, Daras G, Laxa M, Marathias N, Fasseas C, Sweetlove LJ, et al. The role of Lon1 protease in post-germinative growth and maintenance of mitochondrial function in Arabidopsis thaliana. New Phytol 2009; 181:588 - 600
- Unseld M, Marienfeld JR, Brandt P, Brennicke A. The mitochondrial genome of Arabidopsis thaliana contains 57 genes in 366,924 nucleotides. Nat Genet 1997; 15:57 - 61
- Howell KA, Millar AH, Whelan J. Ordered assembly of mitochondria during rice germination begins with pro-mitochondrial structures rich in components of the protein import apparatus. Plant Mol Biol 2006; 60:201 - 223
- Millar AH, Sweetlove LJ, Giege P, Leaver CJ. Analysis of the Arabidopsis mitochondrial proteome. Plant Physiol 2001; 127:1711 - 1727
- Heazlewood JL, Tonti-Filippini JS, Gout AM, Day DA, Whelan J, Millar AH. Experimental analysis of the Arabidopsis mitochondrial proteome highlights signaling and regulatory components, provides assessment of targeting prediction programs, and indicates plant-specific mitochondrial proteins. Plant Cell 2004; 16:241 - 256
- Suzuki CK, Suda K, Wang N, Schatz G. Requirement for the yeast gene LON in intramitochondrial proteolysis and maintenance of respiration. Science 1994; 264:273 - 276
- Logan DC, Millar AH, Sweetlove LJ, Hill SA, Leaver CJ. Mitochondrial biogenesis during germination in maize embryos. Plant Physiol 2001; 125:662 - 672
- Rylott EL, Hooks MA, Graham IA. Co-ordinate regulation of genes involved in storage lipid mobilization in Arabidopsis thaliana. Biochem Soc Trans 2001; 29:283 - 287
- Penfield S, Graham S, Graham IA. Storage reserve mobilization in germinating oilseeds: Arabidopsis as a model system. Biochem Soc Trans 2005; 33:380 - 383
- Smith CK, Baker TA, Sauer RT. Lon and Clp family proteases and chaperones share homologous substrate-recognition domains. Proc Natl Acad Sci USA 1999; 96:6678 - 6682
- Rep M, Nooy J, Guelin E, Grivell LA. Three genes for mitochondrial proteins suppress null-mutations in both AFG3 and RCA1 when overexpressed. Curr Genet 1996; 30:206 - 211
- Savel'ev AS, Novikova LA, Kovaleva IE, Luzikov VN, Neupert W, Langer T. ATP-dependent proteolysis in mitochondria: m-AAA protease and PIM1 protease exert overlapping substrate specificities and cooperate with the mtHsp70-system. J Biol Chem 1998; 273:20596 - 20602