Abstract
Freezing tolerance is an important feature for plant survival during winter. In plants, extracellular freezing occurs at subzero temperatures, resulting in dehydration and mechanical stresses upon the plasma membrane. However, many plants can acquire enhanced freezing tolerance by exposure to non-freezing temperatures, which is referred to as cold acclimation. The plasma membrane is the primary site of freezing injury. During cold acclimation, the lipid composition in the plasma membrane changes, which may protect the plasma membrane from injuries caused by freeze-induced dehydration stress (e.g., phase transition). Recently, we have examined the behavior of the plasma membrane during the freezing process using protoplasts isolated from cold-acclimated Arabidopsis leaves. The observations indicate that the cryobehavior of the plasma membrane after cold acclimation may act to resist the mechanical stress caused by freezing.
Late fall and winter bring subzero temperatures and, consequently, freezing that occurs inside non-thermogenic organisms. Plants that are grown in temperate and frigid zones have to overcome severe conditions without moving or heating themselves. Most of these plants acquire tolerance against freezing by sensing non-freezing low temperatures and changes in day length.Citation1 This is referred to as cold acclimation, during which considerable changes occur in plant cells through gene expression, including increases in the quantity of osmolytes, such as soluble sugars and proline, and alterations in the lipid and protein composition in the plasma membrane.Citation2–Citation6
Freezing tolerance of plants is tightly connected to the mechanism by which plant cells avoid the injuring of the plasma membrane induced by extracellular freezing. Freezing a suspension buffer of protoplasts isolated from leaves to subzero temperatures at which the protoplasts suffer injury causes the protoplasts to become shrunken due to freeze-induced dehydration and they may not recover their original shapes after thawing. This form of injury is termed loss of osmotic responsiveness (LOR). In non-acclimated winter rye, spring oat and Arabidopsis, several electron microscopic studies have revealed that a transition from lamellar (Lα) to hexagonal II (HII) phase in the plasma membrane occurs resulting from close apposition of the plasma membrane and internal endomembranes.Citation4,Citation5,Citation7 Lα-to-HII phase transition is an injury that leads to solute leakage from the cells and consequently results in LOR. In addition, Lα-to-HII phase transition is observed not only in protoplasts but also in non-acclimated winter rye and Arabidopsis leaves.Citation4,Citation5,Citation8
In non-acclimated protoplasts, a different form of freezing injury has been reported. Freezing and osmotic dehydration induce large endocytotic vesicles in protoplasts isolated from non-acclimated winter rye leaves.Citation9 However, endocytotic vesicles have no capability for reincorporation into the plasma membrane during thawing or rehydration and hence protoplasts burst, which is referred to as expansion-induced lysis (EIL).Citation10,Citation11 Thus the biological response of plant cells during freezing results in the injury after thawing. In protoplasts of non-acclimated Arabidopsis leaves, EIL is responsible for 19–28% of freezing injury over the temperature range of −2°C to −4°C.Citation12
The enhanced freezing tolerance in cold-acclimated plants is associated with the resistance mechanism against Lα-to-HII phase transition and EIL, the two forms of freeze-induced injury occurring in non-acclimated protoplasts and cells. The proportion of phosphatidylcholine containing diunsaturated species increases in the plasma membrane during cold acclimation in spring oat, winter rye and Arabidopsis.Citation5,Citation13 The incidence of EIL and Lα-to-HII phase transition is related to lipid composition in the plasma membrane because a similar phenomenon is also observed in reconstructed liposome vesicles, which are composed of lipids similar to those found in the plasma membrane of non-acclimated cells, without any proteins.Citation4 Artificial alternation of lipid composition in the plasma membrane of rye protoplasts by adding diunsaturated species of phosphatidylcholine enhances freezing tolerance as a consequence of decreases in the incidence of EIL and Lα-to-HII phase transition.Citation4 Therefore, the change of lipid composition during cold acclimation is thought to be a mechanism of enhanced freezing tolerance.
Protoplasts isolated from cold-acclimated leaves of winter rye and exposed to a hypertonic solution develop many filiform projections on the surface of the protoplast, referred to as exocytotic extrusions.Citation9,Citation14 Exocytotic extrusions can appear in dehydrated non-acclimated winter rye protoplasts if the proportion of diunsaturated phosphatidylcholine species is artificially increased in the plasma membrane.Citation4 Exocytotic extrusions are believed to enhance the freezing tolerance of cold-acclimated protoplasts, as a means of avoiding EIL, because the extrusions are reversible and can be reincorporated into the plasma membrane during thawing of the suspending buffer.Citation9 However, because exocytotic extrusions can detach from the plasma membrane during osmotic dehydration in some cases,Citation15 the contribution of exocytotic extrusions to freezing tolerance may be less than expected.
Freeze-induced dehydration is a major factor in the injury of the plasma membrane. However, a freezing event produces not only dehydration but also mechanical stress on the plasma membrane. It is expected that ice crystal formation in extracellular freezing results in mechanical stress upon the plasma membrane. Thus it is important to understand how the plasma membrane of cold-acclimated plant cells withstands the mechanical stress that must be directly applied to the plasma membrane.
The lipid bilayer, like the plasma membrane, is a kind of nanomaterial, the physicochemical property of which provides elasticity against strain. On the other hand, the degree of elasticity of the plasma membrane of eukaryotic cells is much higher than the degree of elasticity that is physicochemically expected.Citation16 This issue has been interpreted by the hypothesis of ‘surface area regulation (SAR)’, according to which the elasticity of the plasma membrane of living cells against strain is the product of a biological mechanism(s), rather than the physicochemical property of the lipid bilayer. The SAR hypothesis is briefly described as follows: surface area is added locally from endomembranes caused by increasing the tension of the membrane, and vice versa, a local decrease in plasma membrane tension leads to the localized retrieval of excess surface area.Citation17–Citation19 SAR is thought to be a system to maintain the tension of the plasma membrane at a constant value in eukaryotes, including plants. An example of SAR is reported in molluscan neurons: invaginations of the plasma membrane into the cytoplasm occur as cells shrink during hypertonic treatments, and disappear during hypotonic-induced swelling.Citation17 These are referred to as vacuole-like dilations (VLDs).
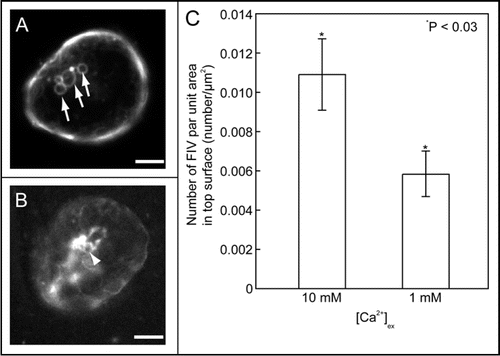
Upon freezing, the plasma membrane suffers physical stresses, which are basically osmotic dehydration and mechanical deformation. Using a lipophilic fluorescent dye, FM1-43, and confocal fluorescence cryomicroscopy, we observed the behavior of the plasma membrane of protoplasts prepared from cold-acclimated Arabidopsis leaves during extracellular freezing.Citation20 The observations showed that freeze-induced vesicular structures (FIVs) appeared immediately after the development of ice crystals contact with the surface of the protoplasts (). Detailed analyses demonstrated that FIVs can be induced by mechanical deformation rather than osmotic dehydration of protoplasts. In the thawing process, FIVs were incorporated into the plasma membrane. Interestingly, the reduction of total area of the plasma membrane during the freezing process was tightly correlated to the enlargement of a single FIV size. Consequently, our results suggest that FIV is a component in SAR during the freezing process, and SAR may contribute to the freezing tolerance by relaxing the mechanical deformation of the plasma membrane. In addition, we found that FIV formation was sometimes unsuccessful at low calcium concentrations, and frequency of FIV appearance was dependent on extracellular calcium concentration ( and C). This suggests that recognition of mechanical stress may use a calcium channel gated by changes of tension in the plasma membrane, which is activated by cold-acclimation.
Because we used a protoplast system to observe the plasma membrane, and the cell wall can be a physical barrier against ice crystals, mechanical stress to intact walled cells may be less than that to protoplasts. Observations of epidermal cells in cold-acclimated Arabidopsis leaves revealed that FIV-like structures seem to appear after freezing, but much less than in protoplasts.Citation20 After thawing, these structures completely disappeared. Therefore, the same mechanism in protoplasts may be driven in intact cells, but the mechanical stress on the plasma membrane is probably less in intact cells than in protoplasts.
Although the mechanism of FIV formation from the plasma membrane and FIV incorporation into the plasma membrane are unclear, conventional endo- and exocytotic processes may be responsible. An observation in growth cones of neural cells shows that the expansion of the plasma membrane may be achieved by a recycling system like the conventional mechanism in the synapse, and contributed by a unique organelle, which is a lumenless membrane and attached to the plasma membrane.Citation21 Molecular components of both processes, known in animals and fungi, seem to be present sufficiently in sequenced plant genomes such as Arabidopsis, and they are involved in several membrane-trafficking processes. Although FIV appearance in our experiment is dependent on the concentration of extracellular calcium, the recognition mechanism for mechanical deformation of the plasma membrane should be investigated because SAR in neuron cells is independent of calcium concentration.Citation22
In conclusion, freezing tolerance of cold-acclimated Arabidopsis may involve the resistance against freeze-induced mechanical stress upon the plasma membrane. Although the major part of freezing tolerance has been thought to be associated with resistance to the freeze-induced osmotic dehydration through alterations in the composition of the plasma membrane, our findings clearly suggest that freezing tolerance is also related to resistance to the mechanical deformation of the plasma membrane by means of the SAR mechanism.
Abbreviations
| SAR | = | surface area regulation |
| FIV | = | freeze-induced vesicular structures |
| EIL | = | expansion-induced lysis |
| LOR | = | loss-of-osmotic responsiveness |
Figures and Tables
Figure 1 Freeze-induced vesicular structures (FIVs) and calcium-dependence of FIVs. Cold-acclimated protoplast suspensions with FM1-43 were frozen from 0°C to −4°C in suspensions at different extracellular calcium concentrations, and FM1-43 fluorescence was then observed at −4°C with a disc-scanning confocal microscope (A, 10 mM; B, 1 mM). Although FIVs were observed in 10 mM calcium (arrow), aberrant FM1-43 fluorescence was observed on the surface area of the protoplast at 1 mM extracellular calcium (arrow head). The numbers of FIVs at these two calcium concentrations were calculated using the confocal images at −4°C (C). This chart shows the number of FIVs per unit surface area of an optical section of the protoplast just before freezing. Bars represent 10 µm. Data are means ± s.e. (n = 16−20).
Acknowledgements
This study was supported by a grant from the 21st Century COE Program to Iwate University (K-03), Grants-in-Aid for Scientific Research (no. 18780242 to Y.K., no. 20780229 to T.Y. and no. 17380062 to M.U.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and the President Fund of Iwate University.
Addendum to:
References
- Levitt J. Responses of Plants to Environmental Stresses (Physiological Ecology): Chilling, freezing and high temperature stresses 1980; Academic Press
- Thomashow MF. PLANT COLD ACCLIMATION: Freezing Tolerance Genes and Regulatory Mechanisms. Annu Rev Plant Physiol Plant Mol Biol 1999; 50:571 - 599
- Hare PD, Cress WA, Van Staden J. Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ 2002; 21:535 - 553
- Steponkus PL, Uemura M, Webb MS. PL S. A contrast of the cryostability of the plasma membrane of winter rye and spring oat—two species that widely differ in their freezing tolerance and plasma membrane lipid composition. Low-temperature biology 1993; London JAI 211 - 312
- Uemura M, Joseph RA, Steponkus PL. Cold Acclimation of Arabidopsis thaliana (Effect on Plasma Membrane Lipid Composition and Freeze-Induced Lesions). Plant Physiol 1995; 109:15 - 30
- Kawamura Y, Uemura M. Mass spectrometric approach for identifying putative plasma membrane proteins of Arabidopsis leaves associated with cold acclimation. Plant J 2003; 36:141 - 154
- Webb MS, Uemura M, Steponkus PL. A comparison of freezing injury in oat and rye: two cereals at the extremes of freezing tolerance. Plant Physiol 1994; 104:467 - 478
- Nagao M, Arakawa K, Takezawa D, Fujikawa S. Long- and short-term freezing induce different types of injury in Arabidopsis thaliana leaf cells. Planta 2008; 227:477 - 489
- Dowgert MF, Steponkus PL. Behavior of the plasma membrane of isolated protoplasts during a freeze-thaw cycle. Plant Physiol 1984; 75:1139 - 1151
- Gordon-Kamm WJ, Steponkus PL. Lamellar-to-hexagonal II phase transitions in the plasma membrane of isolated protoplasts after freeze-induced dehydration. Proc Natl Acad Sci USA 1984; 81:6373 - 6377
- Uemura M, Tominaga Y, Nakagawara C, Shigematsu S, Minami A, Kawamura Y. Responses of the plasma membrane to low temperatures. Physiol Plant 2006; 126:81 - 89
- Kawamura Y, Uemura M. Li PH, Chen TH. Changes in the plasma membrane from Arabidopsis thaliana within 1 week of cold acclimation. Plant Cold Hardiness 2002; New York Plenum Press 181 - 194
- Uemura M, Steponkus PL. A contrast of the plasma membrane lipid composition of oat and rye leaves in relation to freezing tolerance. Plant Physiol 1994; 104:479 - 496
- Steponkus PL, Uemura M, Balsamo RA, Arvinte T, Lynch DV. Transformation of the cryobehavior of rye protoplasts by modification of the plasma membrane lipid composition. Proc Natl Acad Sci USA 1988; 85:9026 - 9030
- Dowgert MF, Wolfe J, Steponkus PL. The mechanics of injury to isolated protoplasts following osmotic contraction and expansion. Plant Physiol 1987; 83:1001 - 1007
- Wolfe J, Steponkus PL. Mechanical properties of the plasma membrane of isolated plant protoplasts: mechanism of hyperosmotic and extracellular freezing injury. Plant Physiol 1983; 71:276 - 285
- Mills LR, Morris CE. Neuronal plasma membrane dynamics evoked by osmomechanical perturbations. J Membr Biol 1998; 166:223 - 235
- Morris CE, Homann U. Cell surface area regulation and membrane tension. J Membr Biol 2001; 179:79 - 102
- Raucher D, Sheetz MP. Membrane expansion increases endocytosis rate during mitosis. J Cell Biol 1999; 144:497 - 506
- Yamazaki T, Kawamura Y, Uemura M. Cryobehavior of the plasma membrane in protoplasts isolated from cold-acclimated arabidopsis leaves is related to surface area regulation. Plant Cell Physiol 2008; 49:944 - 957
- Cheng TP, Reese TS. Recycling of plasmalemma in chick tectal growth cones. J Neurosci 1987; 7:1752 - 1759
- Herring TL, Slotin IM, Baltz JM, Morris CE. Neuronal swelling and surface area regulation: elevated intracellular calcium is not a requirement. Am J Physiol 1998; 274:272 - 281