Abstract
It has long been known that fungal pathogens like Fusarium and Alternaria spp. produce toxins (mycotoxin) to kill plant cells. These mycotoxins have been shown to perturb the plant sphingolipid biosynthesis pathway, resulting in the necrotic cell death of plant cells. A recent study by Shi et al.1 revealed that an increase in the amount of cellular sphingoid bases triggers plant programmed cell death (PCD) through accumulation of reactive oxygen species (ROS). These studies point to the importance of sphingolipids in the regulation of plant cell in disease development as well as in defense responses. In the latest report,2 we showed that serine palmitoyltransferase (SPT), the key enzyme of sphingolipid biosynthesis, regulates not only plant cell death but also defense response against a non-host pathogen, soliciting further studies to elucidate the roles of sphingolipids in plant innate immunity.
Sphingolipids are defined as lipids containing sphingoid bases (1,3-dihydroxy-2-amino-alkane and its derivatives) as a structural backbone, and are essential components of membranes in all eukaryotes. The sphingolipids and their metabolites in yeast and animals are known to be powerful generators of a variety of signals involved not only in maintaining cellular homeostasis but also in stress responses and apoptosis (reviewed in refs. Citation3 and Citation4). Moreover, they are key components of the membrane microdomains called lipid rafts, playing important roles in cellular processes including signal transduction, membrane trafficking, cytoskeletal organization and pathogen entry.Citation5
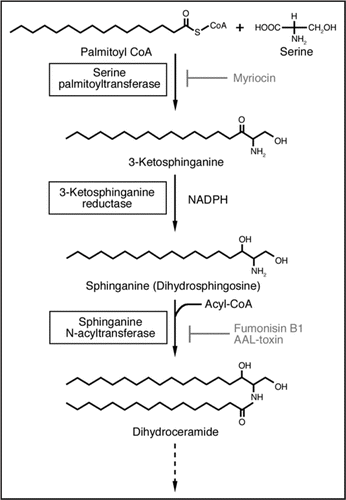
Sphingolipid biosynthesis is initiated by a decarboxyl condensation of serine with palmitoyl-CoA, a reaction catalyzed by serine palmitoyltransferase (SPT) to form 3-ketosphinganine in a pyridoxal 5′-phosphate (PLP) dependent manner. 3-ketosphinganine is immediately converted to sphinganine (dihydrosphingosine) by a 3-ketosphinganine reductase, and sphinganine is converted to dihydroceramide by sphinganine N-acyltransferase (acyl-CoA-dependent ceramide synthase) (). SPT is suggested to be the key enzyme for the regulation of sphingolipid levels in cells.Citation6 In yeast and mammals, SPT is a heterodimer consisting of LCB1 and LCB2 subunits. The PLP binding motif (T[FL][GTS]K[SAG] [FLV]G) around the PLP-binding lysine residue is present in LCB2, but not in LCB1.Citation6 Recently, it has been reported that the LCB1 subunit is involved in the stabilization of the LCB2 protein in yeast and mammalian cells.Citation7,Citation8 Both subunits and the PLP-binding motif in LCB2 are also conserved in plants.Citation9
Several lines of evidence implicate the roles of sphingolipids in plant cell death regulation. Necrotrophic fungi Fusarium moniliforme and Alternaria alternata f sp. lycopersici produce sphinganine-analogous mycotoxins, fumonisin B1 and AAL-toxin, respectively, to kill the host plant via disruption of sphingolipid metabolism resulting from inhibition of sphinganine N-acyltransferase activity ().Citation10–Citation12 AAL-toxin-induced cell death is associated with the accumulation of 3-ketosphinganine and sphinganine in susceptible tomato leaves. This cell death process is blocked by the SPT inhibitor, myriocin, suggesting that the increase in the cellular sphingolipid imetermediates is crucial for the observed cell death.Citation11 The Arabidopsis lesion mimic mutants accelerated cell death (acd) 5 and acd11 exhibiting spontaneous cell death phenotypes were shown to have defects in the genes encoding a putative ceramide kinase and a sphingosine transfer protein, respectively, also pointing to the involvement of sphingolipid intermediates in plant cell death.Citation13,Citation14 Recently Shi et al.Citation1 identified a fumonisin B1-resistant Arabidopsis mutant, fbr11-1, that fails to initiate cell death upon fumonisin B1-treatment. The authors showed that fbr11-1 had a defect in the AtLCB1 gene. Taken together, these observations suggest that an excess of cellular sphingolipids intermediates, more specifically of 3-ketosphinganine and sphinganine, results in plant cell death. Shi et al.Citation1 tested this hypothesis by directly applying free sphingoid bases such as sphinganine, phytosphingosine and sphingosine to plant cells, and showed that the treatment indeed caused reactive oxygen species (ROS) production followed by programmed cell death (PCD)-like cell death. They also suggested that the balance between phosphorylated vs. free (un-phosphorylated) sphingoid bases may determine the fate of plant cell for death or survival.
Our recent study by Takahashi et al.Citation2 supports the previous observations, and further indicated the involvement of sphingolipids in non-host resistance. We performed a high-throughput screening of cell death-inducing factors in plants.Citation15,Citation16 A cDNA library from Nicotiana benthamiana leaves was cloned into a binary potato virus X (PVX)-based expression vector and transformed into Agrobacterium. Individual Agrobacterium colonies were inoculated into N. benthamiana leaves by toothpicks. After 1 to 2 weeks of inoculation, cell death was observed around the inoculated site, if the protein expressed from the integrated cDNA has cell death-inducing activity in plant cells. Using this system, we identified various novel genes from N. benthamiana as cell death-inducing factors,Citation17,Citation18 one of which encoded the LCB2 subunit of SPT (NbLCB2).Citation2 A deletion study indicated that PLP domain is necessary for NbLCB2 to cause cell death. To test the involvement of NbLCB2 in plant resistance against pathogens, we analyzed NbLCB2 transcription following inoculation of plants with the non-host bacterial pathogen Pseudomonas cichorii and the host pathogen Pseudomonas syringae pv. tabaci. During the early stages of infection, NbLCB2 mRNA is strongly induced by P. cichorii, but not by P. syringae pv. tabaci, suggesting the involvement of SPT in non-host resistance. To confirm this finding, we measured pathogen growth in plants whereby SPT function was compromised by the SPT inhibitor myriocin. Resistance of N. benthamiana against P. cichorii was compromised by myriocin treatment, whereas the one against a host pathogen P. syringae pv. tabaci was not affected. Moreover, silencing of NbLCB2 as well as NbLCB1 genes compromised non-host resistance of N. benthamiana against P. cichorii. The combined results suggest that de novo sphingolipid biosynthesis is important for innate immunity against non-host pathogen. Whether this reduction in plant non-host resistance is a consequence of reduction in HR-like cell death or caused by impairment of an as yet unidentified defense signaling pathway remains to be determined.
The details of sphingolipid biosynthesis regulation and the individual function of these molecules are still largely uncharacterized. However, the recent novel findings strongly suggest that sphingolipids play a crucial role in plant innate immunity including cell death. The manipulation of host signaling and the co-option of cell death pathways by plant pathogens by means of their effector molecules (such as AAL toxin or fumonisin) is a fascinating and exciting area of study.Citation19
Figures and Tables
Acknowledgements
This work was carried out in part by support from “Program for Promotion of Basic Research Activities for Innovative Biosciences” (Japan), “Iwate University 21st Century COE Program: Establishment of Thermo-Biosystem Research Program” and MAFF Japan (Genomics for Agricultural Innovation PMI-0010) to R.T., and by “The Sumitomo Foundation” to Y.T. Thanks are due to Matt Shenton and Muluneh Tamiru Oli for improving the manuscript.
Addendum to:
References
- Shi L, Bielawski J, Mu J, Dong H, Teng C, Zhang J, et al. Involvement of sphingoid bases in mediating reactive oxygen intermediate production and programmed cell death in Arabidopsis. Cell Res 2007; 17:1030 - 1040
- Takahashi Y, Berberich T, Kanzaki H, Matsumura H, Saitoh H, Kusano T, et al. Serine palmitoyltransferase, the first step enzyme in sphingolipid biosynthesis, is involved in non-host resistance. Mol Plant-Microbe Interact 2009; In press
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 2008; 9:139 - 150
- Morales A, Lee H, Goñi FM, Kolesnick R, Fernandez-Checa JC. Sphingolipids and cell death. Apoptosis 2007; 12:923 - 939
- Munro S. Lipid rafts: Elusive or illusive?. Cell 2003; 115:377 - 388
- Hanada K. Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim Biophys Acta 2003; 1632:16 - 30
- Gable K, Slife H, Bacikova D, Monaghan E, Dunn TM. Tsc3p is an 80-amino acid protein associated with serine palmitoyltransferase and required for optimal enzyme activity. J Biol Chem 2000; 275:7597 - 7603
- Yasuda S, Nishijima M, Hanada K. Localization, topology and function of the LCB1 subunit of serine palmitoyltransferase in mammalian cells. J Biol Chem 2003; 278:4176 - 4183
- Chen M, Han G, Dietrich CR, Dunn TM, Cahoon EB. The essential nature of sphingolipids in plants as revealed by the functional identification and characterization of the Arabidopsis LCB1 subunit of serine palmitoyltransferase. Plant Cell 2006; 18:3576 - 3593
- Abbas H, Tanaka T, Duke SO, Poter JK, Wray EM, Hodges L, et al. Fumonisin- and AAL-toxin-induced disruption of sphingolipid metabolism with accumulation of free sphingoid bases. Plant Physiol 1994; 106:1085 - 1093
- Spassieva SD, Markham JE, Hille J. The plant disease resistance gene Asc-1 prevents disruption of sphingolipid metabolism during AAL-toxin-induced programmed cell death. Plant J 2002; 32:561 - 572
- Wang H, Li J, Bostock RM, Gilchrist DG. Apoptosis: a functional paradigm for programmed cell death induced by a host-selective phytotoxin and invoked during development. Plant Cell 1996; 8:375 - 391
- Brodersen P, Petersen M, Pike HM, Olszak B, Skov S, Ødum N, et al. Knockout of Arabidopsis ACCELERATED-CELL-DEATH11 encoding a sphingosine transfer prtein causes activation of programmed cell death and defense. Gene Dev 2002; 16:490 - 502
- Liang H, Yao N, Song JT, Luo S, Lu H, Greenberg JT. Ceramides modulate programmed cell death in plants. Gene Dev 2003; 17:2636 - 2641
- Berberich T, Takahashi Y, Saitoh H, Terauchi R. Kahl G, Meksem K. High-throughput functional screening of genes in planta. The Handbook of Plant Functional Genomics 2008; Weinheim WILEY-VCH Verlag GmbH & Co. KGaA 113 - 136
- Terauchi R, Nasir KHB, Ito A, Saitoh H, Berberich T, Takahashi Y. High-throughput functional screening of plant and pathogen genes in planta. Plant Biotech 2005; 22:455 - 459
- Coemans B, Takahashi Y, Berberich T, Ito A, Kanzaki H, Matsumura H, et al. High-throughput in planta expression screening identifies an ADP-ribosylation factor (ARF1) involved in non-host resistance and R gene-mediated resistance. Mol Plant Pathol 2008; 9:25 - 36
- Nasir KHB, Takahashi Y, Ito A, Saitoh H, Matsumura H, Kanzaki H, et al. High-throughput in planta expression screening identifies a class II ethylene-responsive element binding factor-like protein that regulates plant cell death and non-host resistance. Plant J 2005; 43:491 - 505
- Hogenhout SA, van der Hoorn RAL, Terauchi R, Kamoun S. Emerging concepts in effector biology of plant-associated organisms. Mol Plant-Microbe Interact 2009; In press