Abstract
Ethylene is the first identified gaseous hormone regulating many aspects of plant growth and development. ACC and ethephon are two widely used chemicals replacing ethylene treatment when ethylene is not available. However, the amount of ethylene converted by ACC and ethephon is not controllable, leaving it questionable whether either treatment can mimic the effects of ethylene for experiments that are sensitive to ethylene concentration, response window, and treatment durations. Ethylene can be chemically made by ethanol dehydration; however, further purification from the dehydration products is needed. We previously reported that the ethylene gas can be easily prepared by decomposing ethephon in a buffered condition and the resulting ethylene can be used directly. Ethylene responses can be estimated by the measurement of the hypocotyl length of etiolated seedlings, or by ERF1 (Ethylene Response Factor1) expression. Although ACC of low concentrations is insufficient to induce ERF1 expression, ACC of high concentrations can replace ethylene for experiments where ethylene treatment is not feasible. However, ACC may undergo early consumption. Versatile approaches were developed so that laboratories lacking ethylene and techniques for gas handling can easily perform necessary ethylene treatments.
Purity of Ethylene Prepared from Ethephon Decomposition and Ethanol Dehydration
Ethephon (2-chloroethylphosphonic acid) is a widely used chemical replacement for ethylene treatment in many physiological studies.Citation1,Citation2 The conversion of ethephon to ethylene takes place at higher pHs.Citation3 Thus, ethylene can be easily prepared by hydrolyzing ethephon in buffered conditions. For industrial production, ethylene can be prepared by ethanol dehydration; however, the reaction takes place at harsh conditions and further purification is needed.Citation4 The chromatograms for ethylene from a commercial source and prepared from ethephon decomposition are identical and no other hydrocarbon species were detectable (). Gas collected from the ethanol dehydration gives multiple hydrocarbon species that can be detected by the FID (flame ionization detector). In addition to the ethylene and ethanol peaks we previously reported,Citation5 another peak was prominent after the completion of the ethanol dehydration reaction (). Results from GC-MS (Gas chromatography-mass spectrometry) analysis suggest that the hydrocarbon species is diethyl ether (). The production of diethyl ether follows the equation: 2 CH3CH2OH → CH3CH2OCH2CH3 + H2O (on 120°C).
Applications of Ethephon Decomposition in Ethylene Treatment
Ethylene inhibits the hypocotyl elongation in dark-grown seedlings and promotes the chlorophyll degradation in Arabidopsis and in many other plant species.Citation6,Citation7 The seedling triple-response assay and leaf senescence test provide an estimate for extent of ethylene response.Citation6 Although ethylene can be easily prepared in a closed container by ethephon decomposition, it may not be convenient for some non-ethylene laboratories to handle the gas when various ethylene concentrations are needed for physiological treatments. The decomposition of ethephon to ethylene in a buffered condition is rapid, and the reaction nearly completes within 2 days.Citation5 Conceivably, necessary amount of ethylene can be directly produced from ethephon decomposition within a closed container for the seedling triple-response assay and leaf senescence test (). Direct ethylene treatment caused greater extent of leaf senescence than did the ethephon decomposition, possibly due to delayed ethylene release in the ethephon decomposition reaction (). Although ethylene treatment resulted in stronger seedling growth inhibition, the difference was relatively minor and only occurred at low ethylene concentration range (). At higher ethylene concentration (1 µL L−1), there was no difference in the growth inhibition between the two treatments. The minor difference is conceivably caused by delayed ethylene release in the decomposition reaction. The leaf senescence test is a qualitative study and can be performed by directly decomposing necessary amount of ethephon in a closed container. For the seedling triple-response assay, direct ethephon decomposition can replace ethylene treatment when the necessary ethylene concentration is above 0.4 µL L−1.
ERF1 Expression is Induced by High Concentration of ACC
Previously we showed that 10 µM ACC (1-aminocyclopropane-1-carboxylic acid) treatment, a commonly used ACC concentration in many experiments,Citation7,Citation8 was unable to induce ERF1 expression and leaf senescence by foliar spray. However, leaf senescence can be induced by ACC foliar spray when the concentration is extremely high (1 mM) in closed conditions.Citation5 There are experiments where ethylene treatment is not feasible and the use of its replacement will be needed. For example, it is technically challenging to administer ethylene to tissues subjected to experiments involving microscopy. shows that ERF1 expression was highly induced by 1 mM ACC in open and closed conditions. Notably, ERF1 induction dropped quickly 8 hr after treatment in plants treated in open space. In contrast, ERF1 expression gradually increased in plants treated in closed conditions. At the completion of a 12-hr ACC treatment, the final ethylene concentration was 9.5 µL L−1 (), indicating that about 19% of the applied ACC was already converted to ethylene within 12 hr in closed conditions.
This result is in agreement with previous study that ACC undergoes early consumption and that ACC treatment is not ideal for experiments that are sensitive to response window or duration.Citation9 In open conditions, ERF1 induction dropped quickly, implying quicker ACC conversion to ethylene, possibly due to more available oxygen for ACC oxidation. These results provide an explanation for why 1 mM ACC treatment failed to cause leaf senescence in open conditions, albeit ERF1 induction was initially high. For experiments where ethylene treatment is not feasible, such as observing movement or accumulation of GFP-fusion proteins in living cells, ACC of higher concentrations may be used as a replacement.
Figures and Tables
Figure 1A Quality of ethylene prepared from ethephon decomposition and ethanol dehydration. (A) Ethylene from commercial source and ethephon decomposition has identical GC chromatogram.
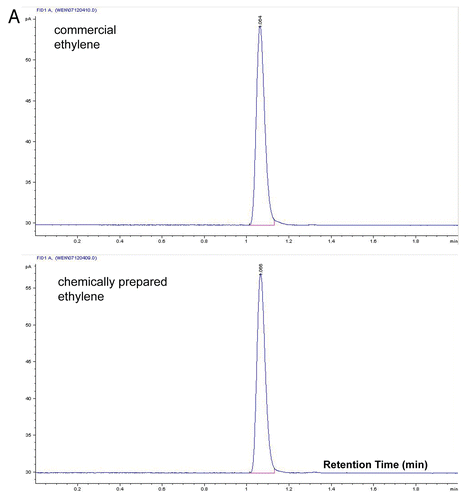
Figure 1B and C Quality of ethylene prepared from ethephon decomposition and ethanol dehydration. (B) Chromatogram of gas species collected from ethanol dehydration by sulfuric acid. (C) Gas species produced by ethanol dehydration were each subjected to MS (mass spectrometry) and ethylene (peak 1), ethanol (peak 2) and diethyl ether (peak 3) are identified.
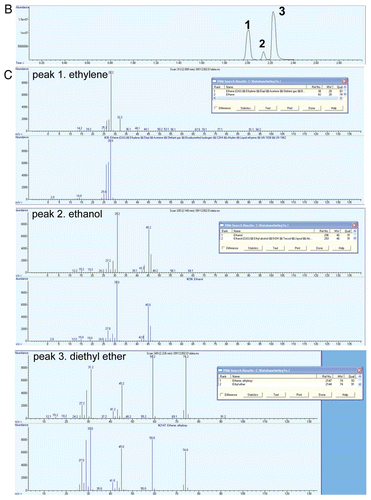
Figure 2 Leaf senescence test and seedling triple-response assay by ethylene directly released from ethephon decomposition without gas handling. (A) Plant materials, seeds or rosette, are placed in a closed container where ethephon decomposition takes place simultaneously. (B) Leaf senescence phenotype for plants respectively treated with ethylene and ethylene directly released from ethephon decomposition. (C) Seedling growth inhibition (80 hr in dark) by ethylene and ethylene released from direct ethephon decomposition.
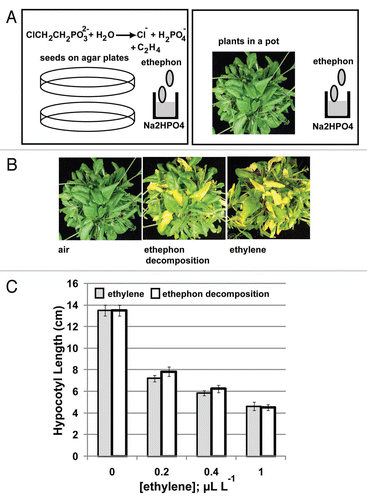
Figure 3 ERF1 expression can be induced by high concentration of Acc foliar spray. (A) ERF1 induction in rosette treated with 1 mM ACC in open space [ACC (open)] and in a closed container [ACC (closed)]. closed: ERF1 expression in rosette in a closed container without ACC treatment. (B) Ethylene evolution in rosette treated with 1 mM ACC in a closed container.
![Figure 3 ERF1 expression can be induced by high concentration of Acc foliar spray. (A) ERF1 induction in rosette treated with 1 mM ACC in open space [ACC (open)] and in a closed container [ACC (closed)]. closed: ERF1 expression in rosette in a closed container without ACC treatment. (B) Ethylene evolution in rosette treated with 1 mM ACC in a closed container.](/cms/asset/707bbf6b-cb62-4db2-8790-e5a35d998ee0/kpsb_a_10910875_f0004.gif)
Addendum to:
References
- Yang SF. Ethylene evolution from 2-chloroethylphosphoate acid. Plant Physiol 1969; 44:1203 - 1204
- Goudey JS, Saini HS, Spencer MS. Uptake and fate of ethephon ([2-chloroethyl] phosphonic acid) in dormant weed seeds. Plant Physiol 1987; 85:155 - 157
- Biddle E, Kerfoot DGS, Kho YH, Russell KE. Kinetic studies of the thermal decomposition of 2-chloroethylphosphonic scid in aqueous solution. Plant Physiol 1976; 58:700 - 702
- Takahara I, Saito M, Inaba M, Murata K. Dehydration of ethanol into ethylene over solid acid catalysts. Catal Letts 2005; 105:249 - 252
- Zhang W, Wen C-K. Preparation of ethylene gas and comparison of ethylene responses induced by ethylene, ACC and ethephon. Plant Physiol Biochem 2010; 48:45 - 53; http://dx.doi.org/10.1016/j.plaphy.2009.10.002
- Hua J, Meyerowitz EM. Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 1998; 94:261 - 271
- Resnick JS, Wen C-K, Shockey JA, Chang C. REVERSION-TO-ETHYLENE SENSITIVITY1, a conserved gene that regulates ethylene receptor function in Arabidopsis. Proc Natl Acad Sci USA 2006; 103:7917 - 7922
- Achard P, Vriezen WH, Van Der Straeten D, Harberd NP. Ethylene regulates Arabidopsis development via the modulation of DELLA protein growth repressor function. Plant Cell 2003; 15:2816 - 2825
- Lavee S, Martin GC. Ethylene evolution following treatment with 1-aminocyclopropane-1-carboxylic acid and ethephon in an in vitro olive shoot system in relation to leaf abscission. Plant Physiol 1981; 67:1204 - 1207