Abstract
Cytokinins are a class of mitogenic plant hormones that influence shoot and root growth, vascular and photomorphogenic development, leaf senescence, and many other aspects of plant growth and development. The Arabidopsis histidine phosphotransfer proteins (AHPs) play an important role in cytokinin signaling by bridging the perception of cytokinins by plasma-membrane receptors to the activation of cytokinin-responsive transcription factors. Based on previous microscopic observations, a model was developed in which the AHPs were thought to relocalize from the cytosol into the nucleus in response to exogenous cytokinin. However, analysis and quantification of the intracellular distribution of AHPs in both protoplasts and intact transgenic plants revealed that the subcellular localization of the AHPs is persistently nucleo-cytosolic and non-responsive to the state of the cytokinin response pathway. Here, we review and extend these findings and discuss their implications.
Cytokinins are a group of plant hormones that play an important role in plant growth and development. The cytokinin signal transduction pathway consists of a two-component phosphorelay signal transduction pathway that conveys extracellular information to the nucleus.Citation1–Citation3 Signaling is initiated when cytokinin binds the extracellular domain of the Arabidopsis histidine kinase (AHKs) receptors. This results in autophosphorylation of the AHKs within their intracellular histidine-kinase domain. Phosphoryl groups are then transferred to cytosolic Arabidopsis histidine phosphotransfer proteins (AHPs), which were suggested to translocate to the nucleus in response to cytokinin treatment. The AHPs then transfer the phosphoryl groups to primarily nuclear-localized response regulators (type-A and type-B ARRs).
Two component response pathways are ubiquitous in prokaryotic cells.Citation4,Citation5 As this signaling module was co-opted by eukaryotic cells, it had to be adapted to the presence of the nuclear envelope and subsequent separation of nuclear and cytosolic signaling components. The AHPs play an important role in accommodating nucleocytosolic separation because they link the plasma membrane-localized receptors to the nuclear-localized response regulators. Previous models for cytokinin signaling suggested that AHP subcellular localization is tied to cytokinin signaling status: AHPs were thought to be localized to the cytosol in unstimulated cells and localized to the nucleus when cells were exposed to exogenous cytokinin.Citation6,Citation7 We investigated the localization of the AHP proteins in protoplasts and intact plants in which the cytokinin response pathway was activated or impaired. We found that bulk AHP subcellular localization is not altered by the status of cytokinin signaling. Instead, we propose that AHP proteins are constantly cycling between the nucleus and cytosol via an active transport mechanism.Citation8
The Overall Distribution of AHPs is Actively Maintained as Nucleo-Cytosolic
To determine the localization of the AHPs in intact plants, we analyzed AHP2-GFP and AHP5-GFP protein fusions driven from native AHP promoters. In all tissues examined, AHPs were found in the nucleus and cytosol. Treatment with exogenous cytokinin did not affect this localization pattern, indicating that subcellular localization of AHPs in planta is independent of cytokinin signaling. In an attempt to repeat the initial observations of AHP movement in response to exogenous cytokinin, we analyzed the localization of AHP1, AHP2 and AHP5 in Arabidopsis mesophyll protoplasts. In wild-type protoplasts, these AHPs were typically found in the both the nucleus and cytosol and its distribution was not altered by treatment with exogenous cytokinins.Citation8 Here, we extended this analysis to include AHP4, whose amino acid sequence is less similar to the other AHP proteins.Citation9 Like all other AHPs tested, AHP4 was typically found in both the nucleus and cytosol, and this pattern was not altered by treatment with trans-zeatin (). We also examined the localization of AHPs in protoplasts obtained from mutant plants defective in upstream components of the cytokinin signaling pathway and again observed that the AHPs were found in both the nucleus and cytosol.Citation8 Finally, to explore the mechanism of nuclear-cytosolic transport, we deleted portions of the AHP proteins and showed that domains within the AHP proteins are responsible for active nuclear import and export.Citation8
Do AHPs Bring Phosphoryl Groups from the Nucleus to the Cytosol?
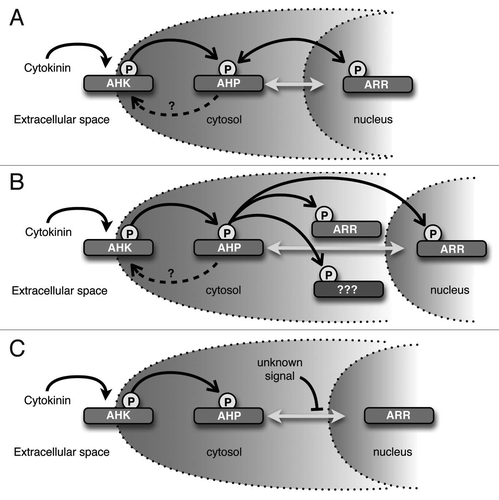
Our results suggest that AHP proteins are constantly cycling between the nucleus and cytosol, which may have interesting biochemical ramifications. The phosphoryl groups that participate in two-component signaling are linked by high-energy bonds, therefore the energy involved in phosphotransfer is close to zero.Citation5,Citation10 Furthermore, the histidine phosphotransfer domains (HPt) of the AHPs do not exhibit catalytic activity, but rather serve essentially as high energy phospho-donors for the receiver domains of the response regulators.Citation4,Citation5 A consequence of this is that phosphotransfer is potentially bidirectional in two-component phosphorelays. One example of such a reverse phosphorelay is the Escherichia coli Arc two-component system that senses anoxic or low redox conditions. In this system, the phosphorylated ArcA response regulator is inactivated via reverse flow of phosphate from ArcA through an Hpt domain to the Asp residue in the receiver domain of the ArcB tripartite sensor kinase receptor.Citation11 In a similar manner, the AHPs could act as phosphatases, playing a role in attenuating cytokinin signaling by removing phosphoryl groups from ARRs when exogenous cytokinin levels are low and potentially shuttling the phosphoryl group back to the Asp residue on the receiver domain of the AHKs. The phosphorylation status of the AHKs, AHPs and ARRs might thus reach equilibrium that is proportional to the concentration of exogenous cytokinin ().
Do Phosphorylated AHPs have a Function in the Cytosol?
Our observation that AHP-GFP fusion proteins are found in the cytosol even upon cytokinin stimulation suggests that phosphorylated AHPs may be present in the cytosol. Several groups have reported that at least some ARR proteins are in part localized to the cytosol.Citation7,Citation12–Citation14 Therefore, another intriguing consequence of our observations is that cytosolic AHPs may transfer phosphoryl groups to cytosolic-localized ARRs (). This also raises the possibility that the AHPs could interact with additional cytoplasmic targets in a phosphor-dependent manner.
Does the AHP Active Transport Mechanism Represent a New Point of Control for Cytokinin Signaling?
Our results indicate that overall AHP subcellular localization is consistently nucleo-cytoplasmic and is not regulated by cytokinin. Interestingly, our data suggest the localization of the AHP proteins appears to be regulated by an unknown active transport mechanism. These observations have several implications for how the cytokinin-signaling pathway could be regulated during plant growth and development. Broadly speaking, this active transport mechanism may be manipulated to control the rate of AHP transport into or out of the nucleus, which in turn, would alter the sensitivity and response to cytokinin (). For example, it is well established that auxin and cytokinin have an antagonistic relationship in many aspects of plant growth and development. An attractive hypothesis is that certain components of the auxin-signaling pathway directly interfere with the kinetics of AHP transport, resulting in an attenuated cytokinin signaling response. Another possibility is that the AHP active transport mechanism is developmentally and/or spatially regulated, resulting in distinct cytokinin sensitivity across different phases of growth or amongst different organs and tissues. Although the cytokinin pathway is arguably the most well characterized plant-hormone signal transduction cascade, the mechanisms modulating intracellular cytokinin response remain to be fully elucidated.
Figures and Tables
Figure 1 Analysis of the subcellular distribution of AHP4-GFP in wild-type Arabidopsis mesophyll protoplasts. (A–D) Representative images of GFP fluorescence signals from protoplasts transfected with plasmids encoding AHP4-GFP fusion proteins that have been treated with either water (control) or 1 µM trans-zeatin for 1 hr. (E) Quantification of data from (A–D). Values are the mean of three independent experiments, error bars indicate SE. Scale bars in (A and C) are 50 µm, scale bars in (B and D) are 25 µm. Methods are as described.Citation8
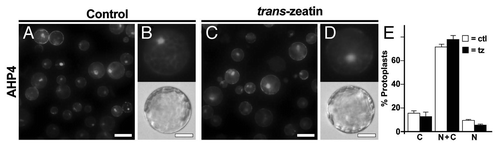
Figure 2 Potential implications of these observations. (A) AHPs may act as phosphatases and remove phosphoryl groups from ARRs. In this model, AHPs would serve to dampen the cytokinin signal if exogenous cytokinin levels were reduced. (B) Cytosolic-localized AHPs may donate phosphoryl groups to cytosolic-localized ARRs or other unkown cytoplasmic targets. (C) Unknown signals could modify AHPs to prevent or enhance AHP entry into the nucleus. As shown, AHPs movement is inhibited, perhaps resulting in reduced sensitivity to cytokinin. Filled arrowheads indicate cytokinin binding, open arrowheads indicate transfer of phosphoryl groups, dashed arrows indicate potential phosphotransfer to AHKs, bidirectional gray arrows indicate AHP movement into and out of the nucleus, and blocked arrowheads indicate inhibition of AHP movement.
Acknowledgements
This work was supported by grants from the United States Department of Agriculture, The Department of Energy, and the National Science Foundation to J.J.K.
Addendum to:
References
- Perilli S, Moubayidin L, Sabatini S. The molecular basis of cytokinin function. Curr Opin Plant Biol 13:21 - 26
- To JPC, Kieber JJ. Cytokinin signaling: two-components and more. Trends Plant Sci 13:85 - 92
- Müller B, Sheen J. Advances in cytokinin signaling. Science 318:68 - 69
- Parkinson J. Signal transuction schemes of bacteria. Cell 73:857 - 871
- Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu Rev Biochem 69:183 - 215
- Hwang I, Sheen J. Two-component circuitry in Arabidopsis signal transduction. Nature 413:383 - 389
- Yamada H, Koizumi N, Nakamichi N, Kiba T, Yamashino T, Mizuno T. Rapid response of Arabidopsis T87 cultured cells to cytokinin through His-to-Asp phosphorelay signal transduction. Biosci Biotechnol Biochem 68:1966 - 1976
- Punwani JA, Hutchison CE, Schaller GEJJK. The subcellular distribution of the Arabidopsis Histidine Phosphotransfer proteins is independent of cytokinin signaling. Plant J 62:473 - 482
- Hutchison CE, Li J, Argueso C, Gonzalez M, Lee E, Lewis MW, et al. The Arabidopsis histidine phospho-transfer proteins are redundant positive regulators of cytokinin signaling. Plant Cell 18:3073 - 3087
- Stock JF, Stock AN, Mottonen JM. Signal transduction in bacteria. Nature 344:395 - 400
- Georgellis D, Kwon O, De Wulf P, Lin EC. Signal decay through a reverse phosphorelay in the Arc two-component signal transduction system. J Biol Chem 273:32864 - 32869
- Sweere U, Eichenberg K, Lohrmann J, Mira-Rodado V, Bäurle I, Kudla J, et al. Interaction of the response regulator ARR4 with the photoreceptor phytochrome B in modulating red light signaling. Science 294:1108 - 1111
- Imamura A, Yoshino Y, Mizuno T. Cellular localization of the signaling components of Arabidopsis His-to-Asp phosphorelay. Biosci Biotechnol Biochem 65:2113 - 2117
- Kiba T, Yamada H, Mizuno T. Characterization of the ARR15 and ARR16 response regulators with special reference to the cytokinin signaling pathway mediated by the AHK4 histidine kinase in roots of Arabidopsis thaliana. Plant Cell Physiol 43:1059 - 1066