Abstract
Arabidopsis has three cytokinin receptors genes: CRE1, AHK2, and AHK3. Availability of plants that are homozygous mutant for these three genes indicates that cytokinin receptors in the haploid cells are dispensable for the development of male and female gametophytes. The triple mutants form a few flowers but never set seed, indicating that reproductive growth is impaired. We investigated which reproductive processes are affected in the triple mutants. Anthers of mutant plants contained fewer pollen grains and did not dehisce. Pollen in the anthers completed the formation of the one vegetative nucleus and the two sperm nuclei, as seen in wild type. The majority of the ovules were abnormal: 78% lacked the embryo sac, 10% carried a female gametophyte that terminated its development before completing three rounds of nuclear division, and about 12% completed three rounds of nuclear division but the gametophytes were smaller than those of the wild type. Reciprocal crosses between the wild type and the triple mutants indicated that pollen from mutant plants did not germinate on wild-type stigmas, and wild-type pollen did not germinate on mutant stigmas. These results suggest that cytokinin receptors in the sporophyte are indispensable for anther dehiscence, pollen maturation, induction of pollen germination by the stigma, and female gametophyte formation and maturation.
Introduction
Cytokinins control a wide range of developmental and physiological processes, including cell proliferation, apical dominance, nutrient mobilization, seed germination, vascular patterning and cambial activity.Citation1,Citation2 However, their functions in sexual reproduction have not been examined in detail.
The female gametophyte is generated from a megaspore, which is formed after meiosis. The megaspore undergoes three rounds of karyokinesis to form an 8-nucleus syncytial cell. This cell, the embryo sac, is then cellularized to form seven cells: an egg, two synergid cells, three antipodal cells and a central cell. In Arabidopsis thaliana, the central cell initially has two nuclei. Female gametophyte formation finishes when the two nuclei of the central cell are fused to form a diploid nucleus.Citation3
A number of genes in female gametophytes are known to be required for their development. SPOROCYTELESS/NOZZLE (SPL/NZZ) is necessary to form cells that undergo meiosis in both carpel and stamen.Citation4,Citation5 Mutations in SWITCH1/DYAD (SWI1/DYAD), which is required for meiosis, impair megaspore formation.Citation6,Citation7 Several mutations are also known to cause developmental arrest during karyokinesis. These include a mutation in genes for anaphase-promoting complex (APC) components NOMEGACitation8 and APC2,Citation9 double mutations in RPT5a and RPT5b, which code for components of the 26S proteasome,Citation10 and double mutations in RHF1a and RHF1b, which code for components of the E3 ubiquitin ligase.Citation11 The slow walker 1 (swa1) mutation delays the mitotic division cycle,Citation12 and the retinoblastoma-related (rbr) mutation causes extra mitotic divisions.Citation13 The ovules of the cytokinin independent1 (cki1) mutants abort after the 4-nucleus stage. As CKI1 is a histidine kinase, phosphorelay is required for female gametophyte development.Citation14,Citation15 GEMINI2/MOR1, which codes for a microtubule-associated protein,Citation16 and TWO IN ONE (TIO), which codes for a phragmoplast-associated protein kinase, are required for the cellularization process.Citation17 An asymmetric auxin gradient in the embryo sac plays a key role in gametic cell specification.Citation18 Finally, LACHESIS (LIS) and CLOTHO/GFA1 (CLO/GFA1) play a central role in gametic cell fate specification.Citation19,Citation20
Several genes in the sporophytes are also known to be indispensable for female gametophyte development. For example, mutants with defective integument initiation and outgrowth, such as ainteguments (ant), inner no outer (ino), bell1 (bel), tousled (tsl) and short integuments1/dicer like1 (sin1/dcl1), are associated with aborted embryo sac development.Citation21 ANT, INO, BEL1, TSL and SIN1/DEL1 code for an AP2-class transcription factor, a YABBY-class transcription factor, a homeodomain-containing transcription factor, a serine/threonine protein kinase and a dicer-like protein, respectively.Citation22–Citation26 These observations suggest that the sporophytic tissue surrounding the female gametophyte plays a role in controlling female gametophyte development.
Formation of the male gametophyte (i.e., the pollen) is initiated by periclinal divisions that form archesporial cells in the anther primordium. Mitotic divisions of the archesporial cells then occur to form the different cell layers: the inner primary sporogenous cells and the outer primary parietal cells. The primary sporogenous cells undergo a small number of divisions to form pollen mother cells, which go through meiotic divisions to form tetrads consisting of four microspores. The microspore undergoes an asymmetric cell division to form the larger vegetative cell and the smaller generative cell. The smaller generative cell again divides to form two sperm cells, which are engulfed in the vegetative cell. The primary parietal cells go through a series of further divisions to form endothecial cells and secondary parietal cells; the secondary parietal cells then divide to form the middle cell layer and the tapetum. After pollen maturation, anthers dehisce to release pollen.Citation27
Mutations that affect each step of pollen development are also known. For example, the spl/nzz mutant fails to form the pollen mother cells and the surrounding cell layers.Citation4,Citation5 EXTRA SPOROGENOUS CELLS/EXCESS MICROSPOROCYTES1 (EXS/EMS1), which encodes a leucine-rich repeat receptor kinase and TAPETAL DETERMINANT1 (TPD1), which encodes for a small secreted protein, may regulate archesporial cell number in the anther.Citation28–Citation32 The sidecar pollen (scp) mutant affects microspore asymmetric division and cellular pattern.Citation33 Mutations in genes for the A-type cyclin-dependent kinase (CDKA;1) and F-box-Like 17 (FBL17) are also known to cause arrest during generative cell division.Citation34,Citation35 DUO POLLEN1 (DUO1), which encodes a R2R3 MYB protein, may function as a generative cell fate determinant, linking cell division and gamete specification.Citation36,Citation37 DYSFUNCTIONAL TAPETUM1 (DYT1), coding for a bHLH transcription factor; ABORTED MICROSPORE (AMS), coding for a bHLH transcriotion factor; and MALE STERILITY 1 (MS1), coding for a PHD-finger transcription factor are required for normal tapetal function and viable pollen production.Citation38
There is some evidence that cytokinins are involved in male reproductive development. For example, anthers of several male-sterile mutants, including the stamenless-2 (sl-2) mutant of tomato (Solanum lycopersicum)Citation39 and a genetic male-sterile line of rapeseed (Brassica napus),Citation40 have lower endogenous cytokinin levels. Cytokinins have also been shown to reverse cytoplasmic male sterility in barley (Hordeum vulgare).Citation41 Accumulation of CKX (cytokinin oxidase/dehydrogenase) in male reproductive tissues of transgenic maize (Zea mays) resulted in male-sterile plants.Citation42 Fertility of Arabidopsis overexpressing AtCKX1 was greatly diminished.Citation43 In rice, the trans-zeatin-type cytokinins were slightly higher in the anther than in the leaf blade and pistil.Citation44
To clarify the role of sporophytic cytokinins in reproductive growth, we carefully examined the phenotypes of a cytokinin-receptor triple mutant (cre1-12 ahk2-2tk ahk3-3), indicating that cytokinin receptors in the sporophyte are required for female gametophyte development and function of pollen and pistil.
Results
Male and female functions are impaired in the cytokinin-receptor triple mutants.
Although a triple mutant containing weaker alleles (ahk2-5 ahk3-7 cre1-2) set a few seeds,Citation45 two other cytokinin-receptor triple mutants (cre1-12 ahk2-2tk ahk3-3, ahk2-1 ahk3-1 ahk4-1) with a stronger phenotype do not produce seeds.Citation46,Citation47 We first examined segregation ratio of the triple-mutant phenotype in a population generated by selfing cre1-12/cre1-12 ahk2-2tk/ahk2-2tk AHK3/ahk3-3 plants (i.e., heterozygous for one of the three cytokinin receptor genes). The triple-mutant phenotype appeared in 24.5% of the plants (147 mutant phenotype: 451 normal phenotype; χ2 = 0.055741, p > 0.05, based on an expected ratio of 1 small: 3 normal), indicating that the presence of the triple-mutant genotype in either male or female gametophytes does not distort the segregation ratio.
The triple mutant plants (cre1-12 ahk2-2tk ahk3-3) were small but occasionally produced an inflorescence with a few flowers. The flowers were smaller than the wild type and looked normal, but did not produce seeds (). This indicates that cytokinin receptors in sporophytic tissue are required for reproductive functions, because the segregation experiment demonstrated that cytokinin receptor genes in gametophytes are dispensable.
To know which male or female functions of the triple mutants are impaired, we performed reciprocal crosses between triple-mutant and wild-type plants. Both male and female functions were impaired in these crosses (). Although the triple mutant female set a few seeds when pollinated with wild-type pollen, those seeds did not germinate. This suggests that female gametophyte functions or pistil functions necessary to support fertilization and embryogenesis are also impaired.
Mutant anthers do not dehisce, and pollen germination is decreased.
To clarify what male processes are impaired, we observed both pollen and anthers in detail. During anther development in Arabidopsis, the male sporophyte and gametophyte tissues undergo unique biological processes, including specific cell divisions (meiosis and division of the haploid nuclei), cell differentiation of the male gametophyte, cell-cell communication between the tapetum and the microspore/pollen, and death of tapetum cells. The process of dehiscence, which involves opening the anther wall to release the mature pollen grains, requires the degeneration of specific anther tissues called the septum and the stomium.Citation48
At anthesis, the stamen filaments of the triple mutants elongated normally ( and C), but the anthers were slightly smaller than the wild type and failed to dehisce ( and B). To analyze the developmental defects in the triple mutant, we compared the terminal phenotypes of anthers in transverse section ( and D). The mutant anthers showed several defects. First, only two- or three-lobed structures were formed in the mutant anthers, whereas four-lobed structures were formed in wild-type anthers. Second, the number of pollen grains was less than that in wild type. Third, degeneration of the tapetum and the break of the septum and stomium were incomplete. Finally, the vascular tissues within the center of the anther failed to form normally.
Despite these defects in anther development, the mutant pollen grains from undehisced anthers were indistinguishable in size and shape from wild type. Both wild-type and mutant pollen grains contained a single vegetative nucleus and two generative nuclei ( and F). To test whether the viability of mutant pollen grains differs from that of wild type, we put both wild-type and mutant pollen grains on artificial germination medium. Pollen from the triple mutant germinated, but at a lower frequency than the wild type, and the pollen tubes were shorter than those of the wild type ( and H).
Female gametophyte development is abnormal in the triple mutant.
To determine the steps of female gametophyte development that were affected by the lack of cytokinin receptors, we observed the megagametophyte terminal phenotypes by using confocal laser scanning microscopy (CLSM).Citation49,Citation50 In Arabidopsis, the megaspore mother cell undergoes meiosis to produce four megaspores in the ovule. Only one functional megaspore out of the four survives, and it then undergoes three rounds of mitotic divisions and subsequent cellularization to produce a seven-celled mature female gametophyte.Citation51,Citation52
All female gametophytes of opened wild-type flowers were at the terminal developmental stage (). We observed 73 ovules of triple-mutant flowers, and the phenotypes of the mutant female gametophytes fell into three categories. In the first and most frequent category (57/73 ovules), the female gametophytes lacked the embryo sac (). In the second category (7/73 ovules), development was terminated before completion () and most female gametophytes had either 2 or 4 nuclei. In the third category (9/73 ovules), the female gametophytes appeared to be morphologically normal (), but the ovules were slightly smaller than the wild type. In the triple mutant, both inner and outer integuments were formed normally, suggesting that the integuments can develop without female gametogenesis.
Pollen-pistil interaction is impaired in the cytokinin receptor triple mutants.
Although a fraction of the female gametophytes appeared to be morphologically normal, the triple mutants were female sterile and produced very few seeds upon pollination with wild-type pollen. Also, although some of the triple-mutant pollen grains germinated in vitro, triple mutant pollen did not produce seed when applied to wild-type pistils (). To investigate whether the sterility is caused by defects in pollen-pistil interaction, we placed pollen from the triple mutant onto wild-type pistils, and wild-type pollen on mutant pistils, and checked for pollen germination. In both cases, the pollen grains did not germinate (), indicating that cytokinin receptors in the sporophyte are required for pollen germination, which depends on pollen-pistil interaction.
Discussion
We have shown that cytokinin receptor genes within the male and female gametophytes are not required for the development of the gametophytes in Arabidopsis. But those in the sporophyte are required for the production of functional gametophytes.
Pollen produced by the triple mutant deficient in the three cytokinin receptors had the usual two sperm nuclei and one vegetative nucleus, and germinated on an artificial medium, albeit with a reduced efficiency. However, these pollen grains did not germinate on wild-type stigmas. The inability to germinate might have been caused by incomplete maturation, because tapetum degeneration was incomplete and anthers did not dehisce. Tapetum degeneration and anther dehiscence normally require jasmonic acid,Citation53 but application of methyl jasmonate (MeJA) to bud clusters had no effect on pollen maturation or anther dehiscence of the cytokinin receptor triple mutant (data not shown). Thus, jasmonic acid and cytokinin independently regulate anther maturation and dehiscence.
In the cytokinin-receptor triple mutant, the integuments, which are sporophytic tissue surrounding the female gametophytes, looked normal. However, a large majority of ovules either lacked female gametophytes altogether or possessed abnormal ones, indicating that cytokinin receptor genes in the sporophyte are required for normal development of the female gametophyte. It is yet to be determined what cytokinin-mediated processes in the sporophyte are required for gametophyte development.
In the triple mutant, the pistil function required to induce pollen germination was impaired. In wild-type plants, stigmatic papillae provide water to pollen in response to stimulus by pollen of the same species; this water then induces pollen germination. It is possible that the triple mutant is deficient in these processes.
Materials and Methods
Plant materials and growth conditions.
Arabidopsis ecotype Columbia was used in all experiments. Plants were grown on plates containing GM medium (MS salts, 1% sucrose, 1/100 vol. of 2.5% MES-KOH at pH 5.7, 0.3% Phytagel) under continuous light at 22°C. The triple-mutant genotype used was cre1-12 ahk2-2tk ahk3-3.Citation46
Microscopy.
Anther structure was examined as described previously in reference Citation29, with the following modifications. Dissected floral buds and inflorescences were fixed in 2.8% (vol/vol) glutaraldehyde in 0.1 M HEPES (N-2-hydroxyethyl piperazine-N′-2-ethanesulfonic acid) buffer (pH 7.2) and 0.02% Triton X-100 overnight at 4°C. Samples were washed twice for 15 min each in 0.1 M HEPES buffer (pH 7.2) and then fixed in 1% OsO4 overnight. Samples were then dehydrated in a graded acetone series and embedded in Spurr's resin. Thin (0.5 µm) sections were made and stained with 1% Toluidine Blue O in 1% H3BO3-NaOH (pH 9.5). For examination of pollen, pollen grains were immersed in a solution of 2 µg/mL 4′,6-diamidino-2-phenylindole and 7% sucrose and viewed by fluorescence microscopy under UV light excitation. For female gametophyte analysis, tissue preparation and microscopy were performed as previously described in reference Citation49 and Citation50, with a slight modification: the pistils fixed in 4% glutaraldehyde, 12.5 mM cacodylate (pH 6.9) and 0.005% Silwet L77 for several hours on ice.
Pollen germination and staining.
In vitro pollen germination and aniline blue staining of pollinated pistils was performed as previously described in reference Citation53.
Figures and Tables
Figure 1 Phenotype of the triple mutant (cre1-12 ahk2-2tk ahk3-3). The triple mutant formed fewer and smaller flowers than wild type. (A) 6-week-old plants of wild type (left) and triple mutant (right). Bars = 1 cm. (B) Wild-type and (C) triple-mutant flowers immediately after opening. Bars = 1 mm.
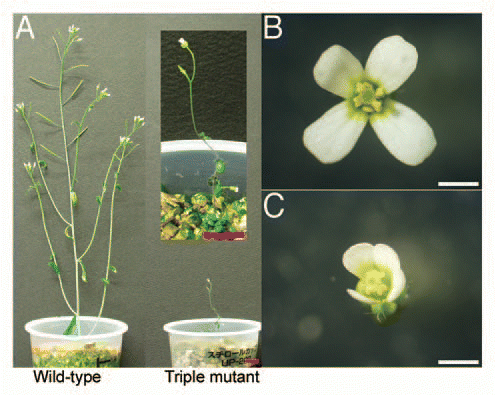
Figure 2 Triple mutant anthers did not dehisce. Pollen of the triple mutant was morphologically normal, but less fraction of pollen, when compared to wild-type, germinated in vitro. (A and B) Anthers removed from open flowers. (A) Wild-type anther. (B) Triple-mutant anther. Bars = 200 µm. (C and D) Transverse sections of anthers. (C) A wild-type anther from a flower immediately before opening. (D) A triple-mutant anther at a similar stage as in (C). In wild-type anthers, the septum (sm) and stomium (st) degenerated, which allow release of pollen grains, whereas in mutant anthers, the septum and stomium did not degenerate. PG, pollen grain. Bars = 50 µm. (E and F) Pollen grains stained with 4′,6-diamidino-2-phenylindole. One vegetative (v) and two generative (g) nuclei were observed in both wild-type (E) and triple-mutant (F) pollen grains. Bars = 10 µm. (G and H) In vitro germination of pollen. (G) Wild type. (H) Triple mutant. Triple-mutant grains germinated less frequently than wild-type, and germinating pollen tubes were shorter than those of wild type. Bars = 50 µm.
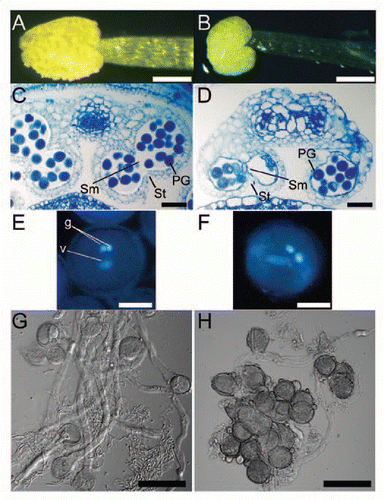
Figure 3 CLSM (confocal laser scanning microscopy) images of mutant and wild-type female gametophytes. The majority of triple-mutant ovules were abnormal. (A) Schematic diagram of ovule, with female gametophyte encircled by red line. (B) Wild-type ovule. (C–E) Triple-mutant ovules. Of the 73 triple-mutant ovules observed, 57 lacked the embryo sac (C), 7 carried a female gametophyte that terminated its development before completion (D), and 9 appeared to be morphologically normal (E). ecn, egg cell nucleus; scn, synergid cell nucleus; sen, secondary nucleus. Bars = 20 µm.
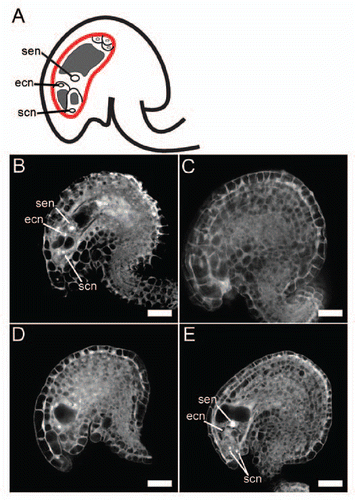
Figure 4 Aniline blue-stained pistils 24 h after reciprocal crosses between wild-type and triple-mutant plants. (A) Wild-type stigma pollinated with wild-type pollen. (B) Wild-type stigma pollinated with triple-mutant pollen. (C) Triple-mutant stigma pollinated with wild-type pollen. White on the stigma (arrow, A) indicates pollen germination. In contrast, no signal was observed in (B and C). pt, pollen tubes; v, vasculature. Bars = 50 µm.
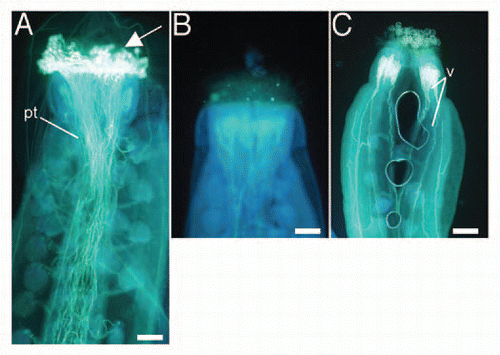
Table 1 Reciprocal crosses between wild-type and triple mutants indicated that male and female functions were impaired
Acknowledgements
We thank Masayuki Higuchi for plant materials. This work was supported by Grants-in-Aid for Scientific Research (KAKENHI) (Grant No. 19060005).
References
- Kakimoto T. Perception and signal transduction of cytokinins. Annu Rev Plant Biol 2003; 54:605 - 627
- Matsumoto-Kitano M, Kusumoto T, Tarkowski P, Kinoshita-Tsujimura K, Vaclavikova K, Miyawaki K, et al. Cytokinins are central regulators of cambial activity. Proc Natl Acad Sci USA 2008; 105:20027 - 20031
- Yang WC, Shi DQ, Chen YH. Female gametophyte development in flowering plants. Annu Rev Plant Biol 2010; 61:89 - 108
- Yang WC, Ye D, Xu J, Sundaresan V. The SPOROCYTELESS gene of Arabidopsis is required for initiation of sporogenesis and encodes a novel nuclear protein. Genes Dev 1999; 13:2108 - 2117
- Schiefthaler U, Balasubramanian S, Sieber P, Chevalier D, Wisman E, Schneitz K. Molecular analysis of NOZZLE, a gene involved in pattern formation and early sporogenesis during sex organ development in Arabidopsis thaliana. Proc Natl Acad Sci USA 1999; 96:11664 - 11669
- Ravi M, Marimuthu MP, Siddiqi I. Gamete formation without meiosis in Arabidopsis. Nature 2008; 451:1121 - 1124
- Mercier R, Vezon D, Bullier E, Motamayor JC, Sellier A, Lefevre F, et al. SWITCH1 (SWI1): a novel protein required for the establishment of sister chromatid cohesion and for bivalent formation at meiosis. Genes Dev 2001; 15:1859 - 1871
- Kwee HS, Sundaresan V. The NOMEGA gene required for female gametophyte development encodes the putative APC6/CDC16 component of the anaphase promoting complex in Arabidopsis. Plant J 2003; 36:853 - 866
- Capron A, Serralbo O, Fulop K, Frugier F, Parmentier Y, Dong A, et al. The Arabidopsis anaphase-promoting complex or cyclosome: molecular and genetic characterization of the APC2 subunit. Plant Cell 2003; 15:2370 - 2382
- Gallois JL, Guyon-Debast A, Lecureuil A, Vezon D, Carpentier V, Bonhomme S, et al. The Arabidopsis proteasome RPT5 subunits are essential for gametophyte development and show accession-dependent redundancy. Plant Cell 2009; 21:442 - 459
- Liu J, Zhang Y, Qin G, Tsuge T, Sakaguchi N, Luo G, et al. Targeted degradation of the cyclin-dependent kinase inhibitor ICK4/KRP6 by RING-type E3 ligases is essential for mitotic cell cycle progression during Arabidopsis gametogenesis. Plant Cell 2008; 20:1538 - 1554
- Shi DQ, Liu J, Xiang YH, Ye D, Sundaresan V, Yang WC. SLOW WALKER1, essential for gametogenesis in Arabidopsis, encodes a WD40 protein involved in 18S ribosomal RNA biogenesis. Plant Cell 2005; 17:2340 - 2354
- Ebel C, Mariconti L, Gruissem W. Plant retinoblastoma homologues control nuclear proliferation in the female gametophyte. Nature 2004; 429:776 - 780
- Hejatko J, Pernisova M, Eneva T, Palme K, Brzobohaty B. The putative sensor histidine kinase CKI1 is involved in female gametophyte development in Arabidopsis. Mol Genet Genomics 2003; 269:443 - 453
- Pischke MS, Jones LG, Otsuga D, Fernandez DE, Drews GN, Sussman MR. An Arabidopsis histidine kinase is essential for megagametogenesis. Proc Natl Acad Sci USA 2002; 99:15800 - 15805
- Glab N, Teste MA, Slonimski PP. MRG1-1, a dominant allele that confers methomyl resistance in yeast expressing the cytoplasmic male sterility T-urf13 gene from maize. Curr Genet 1994; 26:477 - 485
- Palopoli MF, Wu CI. Genetics of hybrid male sterility between drosophila sibling species: a complex web of epistasis is revealed in interspecific studies. Genetics 1994; 138:329 - 341
- Pagnussat GC, Alandete-Saez M, Bowman JL, Sundaresan V. Auxin-dependent patterning and gamete specification in the Arabidopsis female gametophyte. Science 2009; 324:1684 - 1689
- Gross-Hardt R, Kagi C, Baumann N, Moore JM, Baskar R, Gagliano WB, et al. LACHESIS restricts gametic cell fate in the female gametophyte of Arabidopsis. PLoS Biol 2007; 5:47
- Moll C, von Lyncker L, Zimmermann S, Kagi C, Baumann N, Twell D, et al. CLO/GFA1 and ATO are novel regulators of gametic cell fate in plants. Plant J 2008; 56:913 - 921
- Schneitz K. The molecular and genetic control of ovule development. Curr Opin Plant Biol 1999; 2:13 - 17
- Elliott RC, Betzner AS, Huttner E, Oakes MP, Tucker WQ, Gerentes D, et al. AINTEGUMENTA, an APETALA2-like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. Plant Cell 1996; 8:155 - 168
- Villanueva JM, Broadhvest J, Hauser BA, Meister RJ, Schneitz K, Gasser CS. INNER NO OUTER regulates abaxial-adaxial patterning in Arabidopsis ovules. Genes Dev 1999; 13:3160 - 3169
- Reiser L, Modrusan Z, Margossian L, Samach A, Ohad N, Haughn GW, et al. The BELL1 gene encodes a homeodomain protein involved in pattern formation in the Arabidopsis ovule primordium. Cell 1995; 83:735 - 742
- Roe JL, Rivin CJ, Sessions RA, Feldmann KA, Zambryski PC. The Tousled gene in A. thaliana encodes a protein kinase homolog that is required for leaf and flower development. Cell 1993; 75:939 - 950
- Golden TA, Schauer SE, Lang JD, Pien S, Mushegian AR, Grossniklaus U, et al. SHORT INTEGUMENTS1/SUSPENSOR1/CARPEL FACTORY, a Dicer homolog, is a maternal effect gene required for embryo development in Arabidopsis. Plant Physiol 2002; 130:808 - 822
- Wilson ZA, Zhang DB. From Arabidopsis to rice: pathways in pollen development. J Exp Bot 2009; 60:1479 - 1492
- Canales C, Bhatt AM, Scott R, Dickinson H. EXS, a putative LRR receptor kinase, regulates male germline cell number and tapetal identity and promotes seed development in Arabidopsis. Curr Biol 2002; 12:1718 - 1727
- Zhao DZ, Wang GF, Speal B, Ma H. The excess microsporocytes1 gene encodes a putative leucine-rich repeat receptor protein kinase that controls somatic and reproductive cell fates in the Arabidopsis anther. Genes Dev 2002; 16:2021 - 2031
- Yang SL, Xie LF, Mao HZ, Puah CS, Yang WC, Jiang L, et al. Tapetum determinant1 is required for cell specialization in the Arabidopsis anther. Plant Cell 2003; 15:2792 - 2804
- Yang SL, Jiang L, Puah CS, Xie LF, Zhang XQ, Chen LQ, et al. Overexpression of TAPETUM DETERMINANT1 alters the cell fates in the Arabidopsis carpel and tapetum via genetic interaction with excess microsporocytes1/extra sporogenous cells. Plant Physiol 2005; 139:186 - 191
- Jia G, Liu X, Owen HA, Zhao D. Signaling of cell fate determination by the TPD1 small protein and EMS1 receptor kinase. Proc Natl Acad Sci USA 2008; 105:2220 - 2225
- Chen YC, McCormick S. sidecar pollen, an Arabidopsis thaliana male gametophytic mutant with aberrant cell divisions during pollen development. Development 1996; 122:3243 - 3253
- Iwakawa H, Shinmyo A, Sekine M. Arabidopsis CDKA;1, a cdc2 homologue, controls proliferation of generative cells in male gametogenesis. Plant J 2006; 45:819 - 831
- Nowack MK, Grini PE, Jakoby MJ, Lafos M, Koncz C, Schnittger A. A positive signal from the fertilization of the egg cell sets off endosperm proliferation in angiosperm embryogenesis. Nat Genet 2006; 38:63 - 67
- Durbarry A, Vizir I, Twell D. Male germ line development in Arabidopsis. duo pollen mutants reveal gametophytic regulators of generative cell cycle progression. Plant Physiol 2005; 137:297 - 307
- Rotman N, Durbarry A, Wardle A, Yang WC, Chaboud A, Faure JE, et al. A novel class of MYB factors controls sperm-cell formation in plants. Curr Biol 2005; 15:244 - 248
- Zhang W, Sun Y, Timofejeva L, Chen C, Grossniklaus U, Ma H. Regulation of Arabidopsis tapetum development and function by DYSFUNCTIONAL TAPETUM1 (DYT1) encoding a putative bHLH transcription factor. Development 2006; 133:3085 - 3095
- Sawhney VK, Shukla A. Male Sterility In Flowering Plants: Are Plant Growth Substances Involved?. American Journal of Botany 1994; 81:1640 - 1647
- Shukla A, Sawhney VK. Metabolism of Dihydrozeatin in Floral Buds of Wild-Type and a Genie Male Sterile Line of Rapeseed (Brassica napus L.). J Exper Bot 1993; 44:1497 - 1505
- Ahokas H. Cytoplasmic male sterility in barley: Evidence for the involvement of cytokinins in fertility restoration. Proc NatL Acad Sci USA 1982; 79:7605 - 7608
- Huang S, Cerny RE, Qi Y, Bhat D, Aydt CM, Hanson DD, et al. Transgenic studies on the involvement of cytokinin and gibberellin in male development. Plant Physiol 2003; 131:1270 - 1282
- Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmulling T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 2003; 15:2532 - 2550
- Hirano K, Aya K, Hobo T, Sakakibara H, Kojima M, Shim RA, et al. Comprehensive transcriptome analysis of phytohormone biosynthesis and signaling genes in microspore/pollen and tapetum of rice. Plant Cell Physiol 2008; 49:1429 - 1450
- Riefler M, Novak O, Strnad M, Schmulling T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development and cytokinin metabolism. Plant Cell 2006; 18:40 - 54
- Higuchi M, Pischke MS, Mahonen AP, Miyawaki K, Hashimoto Y, Seki M, et al. In planta functions of the Arabidopsis cytokinin receptor family. Proc Natl Acad Sci USA 2004; 101:8821 - 8826
- Nishimura C, Ohashi Y, Sato S, Kato T, Tabata S, Ueguchi C. Histidine kinase homologs that act as cytokinin receptors possess overlapping functions in the regulation of shoot and root growth in Arabidopsis. Plant Cell 2004; 16:1365 - 1377
- Ma H. Molecular genetic analyses of microsporogenesis and microgametogenesis in flowering plants. Annu Rev Plant Biol 2005; 56:393 - 434
- Christensen CA, King EJ, Jordan JR, Drews GN. Megagametogenesis in Arabidopsis wild type and the Gf mutant. Sexual Plant Reproduction 1997; 10:49 - 64
- Christensen CA, Subramanian S, Drews GN. Identification of gametophytic mutations affecting female gametophyte development in Arabidopsis. Dev Biol 1998; 202:136 - 151
- Yang WC, Sundaresan V. Genetics of gametophyte biogenesis in Arabidopsis. Curr Opin Plant Biol 2000; 3:53 - 57
- Drews GN, Yadegari R. Development and function of the angiosperm female gametophyte. Annu Rev Genet 2002; 36:99 - 124
- Ishiguro S, Kawai-Oda A, Ueda J, Nishida I, Okada K. The DEFECTIVE IN ANTHER DEHISCIENCE gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence and flower opening in Arabidopsis. Plant Cell 2001; 13:2191 - 2209