Abstract
Glutathione (GSH) is a low molecular weight thiol compound that plays many roles in photosynthetic organisms. We utilized a ∆gshB (glutathione synthetase) mutant strain as a tool to evaluate the role of GSH in the cyanobacterium Synechocystis sp. PCC 6803 (hereafter Synechocystis 6803), a model photosynthetic organism. The ∆gshB mutant does not synthesize glutathione, but instead accumulates the GSH precursor, gamma-glutamylcysteine (gamma-EC), to millimolar levels. We found that gamma-EC was sufficient to permit cellular proliferation during optimal conditions, but not when cells were exposed to conditions promoting oxidative stress. Furthermore, we found that many factors affecting growth rate and photosynthetic activities strongly influenced cellular thiol content. Here, we are providing some additional insights into the role of GSH and gamma-EC in Synechocystis 6803 during conditions promoting oxidative stress. p.p1 {margin: 0.0px 0.0px 0.0px 0.0px; text-align: justify; font: 10.0px Symbol}
Response of the ΔgshB Strain to Heavy Metals
GSH has been shown to be important for metal tolerance in diverse organisms including bacteria and plants.Citation1,Citation2 GSH has also been found to play important roles in detoxification of cadmium in bacteria and eukaryotic organisms.Citation3,Citation4 We utilized the ΔgshB strain as a tool to probe the effect of several metals on the cellular physiology Synechocystis 6803 in the absence of GSH.
We found that the ΔgshB strain is extremely sensitive to growth in the presence of CdCl2 compared to the wild-type (WT) strain (), similar to findings with Escherichia coli and yeast.Citation3,Citation4 In Synechocystis 6803, cadmium elicits many similar responses to that of H2O2, resulting in the downregulation of processes involved in energy metabolism and upregulation of processes involved in iron homeostasis and oxidative stress.Citation5 We have previously concluded that reactive oxygen species (ROS) are generated during many environmental perturbations.Citation6 Moreover, recent work has implicated GSH and the glutaredoxin system in selenateCitation7 and arsenateCitation8 detoxification in Synechocystis 6803. However, the role of GSH as an electron donor in these pathways in vivo remains unclear. Marteyn et al. have shown that Grx1 and Grx2 function in a electron transfer cascade to reduce selenate.Citation7 We found that the ΔgshB strain behaves similar to WT during growth in the presence of NaSeO4 at concentrations as high as 20 µM, with both strains exhibiting reduced growth. Similarly, we did not observe a significantly enhanced sensitivity of the ΔgshB strain compared to WT in phosphate deplete BG11 medium containing 100 mM Na-AsV. Taken together, these data suggest that GSH, per se is not required for defense against arsenate and selenate in Synechocystis 6803. Further, they support the notion that while Grx1 and Grx2 have GSH-dependent disulfide oxidoreductase activity, GSH is likely not required for their function in vivo as they are able to receive electrons from NADPH-dependent thioredoxin reductase and a ferredoxin.Citation7 It is also possible that γ-EC, if present at high concentrations, is able to functionally replace GSH.Citation9
Insights into the Maintenance of Redox Homeostasis in Synechocystis 6803
To characterize the physiology of Synechocystis 6803 strains, we routinely assay their photosynthetic activities using spectroscopic and biochemical methods. We commonly utilize a Clark-type electrode to directly measure dissolved oxygen concentrations, the byproduct of photosynthetic water splitting and substrate of respiration and Mehler reactions. Specifically, using artificial electron donor/acceptor pairs, we can characterize the activities of photosystem I (PSI) and photosystem II (PSII) complexes as a function of light intensity.
We were somewhat surprised to determine that the ΔgshB strain exhibited extremely low PSI-mediated electron transport activity in intact cell assays using ascorbate/3,6-diaminodurene (DAD) as the electron donors and methyl viologen (MV) as the electron acceptor (). In this assay, we first measure respiratory consumption of oxygen in the dark as a baseline, and then illuminate the samples with varying light intensity to determine the net oxygen consumption due to electron flow through PSI. However, previous measurements of whole chain oxygen evolution (H2O to CO2) and PSII measurements using 1 mM potassium ferricyanide and 0.5 mM 2,6-dichloro-p-benzoquinone did not indicate a deficiency in electron transport.
To further investigate this phenomenon, we isolated membrane fractions from WT, ΔgshB and the complemented ΔgshB/T2086 strains, and assayed their PSI-mediated electron transfer reactions at saturating light intensity (8,250 µmolphotonsm−2s−1) as previously described in reference Citation10 (). We consistently found, using several sets of independently isolated membranes, that the activity of the ΔgshB membranes was significantly enhanced compared to the WT and ΔgshB/T2086 strains in an apparent contradiction to the whole cell data.
We would therefore like to speculate about the nature of these findings. We previously found that the ΔgshB strain is extremely sensitive to growth in the presence of MV at concentrations >0.5 µM.Citation11 The PSI assay utilizes MV at concentrations significantly higher than this (2 mM), however, the overall duration of the measurement is in minutes. It is possible that in this time frame, sufficient reactive oxygen species (ROS) are generated to damage the functional PSI complexes and prevent accurate measurement of the activity. Studies on a cyanobacterial superoxide dismutase mutant claim that MV primarily damages PSI compared to PSII.Citation12 While less studied than PSII photoinhibition, PSI photoinhibition has been observed in plants following cold treatment, and ROS mediated loss of Fe-S clusters is the likely cause.Citation13,Citation14 In the WT strain, it is possible that endogenous ROS scavenging mechanisms are sufficient to overcome the amount of ROS produced and artificial electron transfer through PSI can proceed. Membranes were measured in a buffered reaction mix containing superoxide dismutase as a scavenger of the superoxide anion radical formed upon single electron reduction of O2. While superoxide is not likely the direct cause of PSI inactivation, Fenton reactions involving the product of superoxide dismutation, H2O2 and Fe-S clusters of PSI result in formation of the hydroxyl radical that will inactivate PSI.Citation14 In this artificial system using membranes, we are able to observe PSI mediated electron transport, though it has been found that methyl viologen is able to accept electron from damaged PSI complexes.Citation15
The nature of the enhanced activity of the ΔgshB membranes is currently not known. We did not find significant differences in the amounts of the PSI P700 reaction centers in the ΔgshB mutant compared to WT. It is possible that damage or alterations to the donor side of PSI could increase MV access sites of electron transfer components, thereby enhancing the apparent rate of reaction, however we have no data to support this in our case. Additionally, we cannot rule out the possibility that other components of the photosynthetic electron transport chain contribute to oxygen reduction in this assay.Citation16
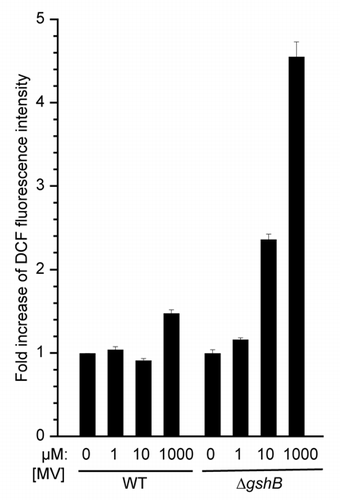
To test the hypothesis that increased ROS generation is responsible for the observed low PSI-mediated electron transport activity in the ΔgshB strain, we utilized the fluorescent ROS indicator 2′,7′-dichlorofluoresceine diacetate as described in reference Citation11, to monitor ROS production in cells following 30 minutes of MV treatment at varying concentrations (). At 1 mM MV, the WT strain exhibited <2-fold increase in fluorescence compared to the untreated, while the ΔgshB strain exhibited a >4-fold increase compared to the control. These data suggest that increased ROS production in cells upon MV treatment could lead to the inhibition of the PSI mediated electron transport in the ΔgshB strain.
These results highlight the multi-functionality of GSH in Synechocystis 6803. While γ-EC is able to function under ideal conditions, it is not sufficient under conditions that greatly disturb the cellular redox balance. It is interesting to note, however, that γ-EC is the primary thiol in halobacteria.Citation17 Therefore it is likely that the subtle differences between these molecules and the co-evolution of the intricate pathways they participate in will dictate the cellular responses to many environmental perturbations.
Figures and Tables
Figure 1 Growth of WT (circles) and the ΔgshB strain (squares) in the absence (open symbols) or presence of 10 µM CdCl2 (closed symbols). Growth was measured as optical density (OD) at 730 nm on a microtiter plate spectrophotometer (BioTek, Winooksi, VT).
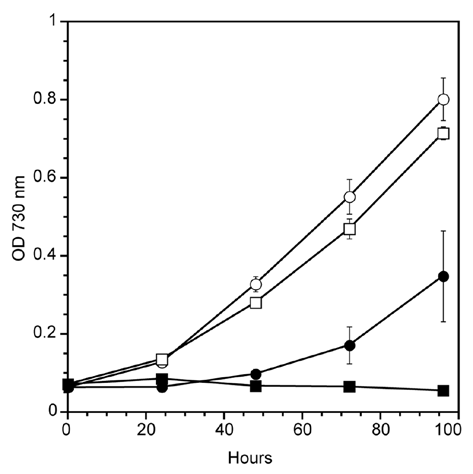
Figure 2 Photosystem I-mediated electron transport in Synechocystis 6803 strains. Oxygen consumption was monitored on a Clark-type electrode in whole cells (A) or in isolated membranes (B) adjusted to a chlorophyll concentration of 5 µg/ml. WT (circle, line) and ΔgshB (square, dashed) cells were suspended in BG11 medium and measured in the presence of 20 µM 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU ), 1 mM sodium ascorbate, 2 mM methyl viologen and 1 mM 3,6-diaminodurene at varying light intensities using neutral density filters (A). (B) Membranes from WT, ΔgshB and the complemented ΔgshB/T2086 strain were harvested from cells following breakage and centrifugation. Oxygen consumption was measured at a saturating light intensity of 8,250 µmol photons m−2 s−1 red light. Membranes were suspended in measuring buffer consisting of 50 mM Hepes (pH 7.5), 10 mM NaCl, 5 mM MgCl2, 1 mM KCN , 20 µM DCMU, 10 µg/ml superoxide dismutase (Sigma), 0.1 mM dichlorophenolindolphenol and 1 mM methyl viologen (B). Error bars represent SE of three measurements for whole cells and from three independent membrane preparations for WT and ΔgshB. The ΔgshB/T2086 value represents a single measurement.
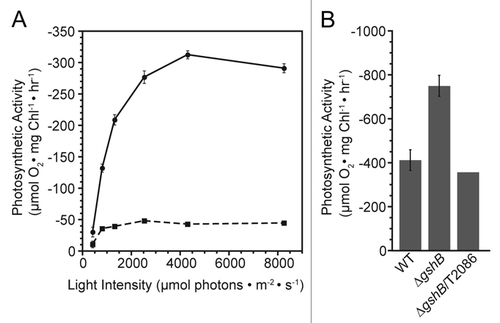
Figure 3 Estimation of cellular ROS after treatment with methyl viologen (MV). WT and the ΔgshB strains were incubated at 30°C and illuminated with 30 µmol photons m−2 s−1 for 0.5 h with varying amounts of MV. 2′,7′-dichlorofluoresceine diacetate (DCF; Sigma) was added to cells at a concentration of 10 µM. Fluorescence intensity at 525 nm was monitored following 488 excitation using a Synergy Mx fluorescence platereader (Biotek, Winooski, VT). Fluorescence intensity was normalized to cell density and is represented as fold increase compared to control (0 µM MV). Error bars represent standard error of four measurements.
Acknowledgements
This work was supported by funding from the National Science Foundation-FIBR program (EF0425749), and also from the Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences, Office of Science, US Department of Energy (DE-FG02-09ER20350).
Addendum to:
References
- Howden R, Andersen CR, Goldsbrough PB, Cobbett CS. A cadmium-sensitive, glutathione-deficient mutant of Arabidopsis thaliana. Plant Physiol 1995; 107:1067 - 1073
- Helbig K, Bleuel C, Krauss G, Nies D. Glutathione and transition metal homeostasis in Escherichia coli. J Bacteriol 2008; 190:5431 - 5438
- Helbig K, Grosse C, Nies D. Cadmium toxicity in glutathione mutants of Escherichia coli. J Bacteriol 2008; 190:5439 - 5454
- Preveral S, Gayet L, Moldes C, Hoffmann J, Mounicou S, Gruet A, et al. A common highly conserved cadmium detoxification mechanism from bacteria to humans: heavy metal tolerance conferred by the ATP-binding cassette (ABC) transporter SpHMT1 requires glutathione but not metal-chelating phytochelatin peptides. J Biol Chem 2009; 284:4936 - 4943
- Houot L, Floutier M, Marteyn B, Michaut M, Picciocchi A, Legrain P, et al. Cadmium triggers an integrated reprogramming of the metabolism of Synechocystis PCC6803, under the control of the Slr1738 regulator. BMC Genomics 2007; 8:350
- Singh AK, Elvitigala T, Cameron JC, Ghosh BK, Bhattacharyya-Pakrasi M, Pakrasi HB. Integrative analysis of large scale expression profiles reveals core transcriptional response and coordination between multiple cellular processes in a cyanobacterium. BMC Syst Biol 2010; 4:105
- Marteyn B, Domain F, Legrain P, Chauvat F, Cassier-Chauvat C. The thioredoxin eductase-glutaredoxins-ferredoxin crossroad pathway for selenate tolerance in Synechocystis PCC6803. Mol Microbiol 2008; 71:520 - 532
- Lopez-Maury L, Sanchez-Riego A, Reyes J, Florencio F. Glutathione/glutaredoxin system is essential for arsenate reduction in Synechocystis sp. PCC 6803. J Bacteriol 2009; 191:3534 - 3543
- Bick JA, Aslund F, Chen Y, Leustek T. Glutaredoxin function for the carboxyl-terminal domain of the plant-type 5′-adenylylsulfate reductase. Proc Natl Acad Sci USA 1998; 95:8404 - 8409
- Mannan R, Pakrasi H. Dark heterotrophic growth conditions result in an increase in the content of photosystem II units in the filamentous cyanobacterium Anabaena variabilis ATCC 29413. Plant Physiol 1993; 103:971 - 977
- Cameron JC, Pakrasi HB. Essential role of glutathione in acclimation to environmental and redox perturbations in the cyanobacterium Synechocystis sp. PCC 6803. Plant Physiol 2010; 154:1672 - 1685
- Herbert SK, Samson G, Fork DC, Laudenbach DE. Characterization of damage to photosystems I and II in a cyanobacterium lacking detectable iron superoxide dismutase activity. Proc Natl Acad Sci USA 1992; 89:8716 - 8720
- Sonoike K. Photoinhibition of photosystem I: its physiological significance in the chilling sensitivity of plants. Plant Cell Physiol 1996; 37:239 - 247
- Sonoike K. Photoinhibition and protection of photosystem I. Adv Photosynth Resp 2006; 24:657 - 668
- Terashima I, Funayama S, Sonoike K. The site of photoinhibition in leaves of Cucumis sativus L. at low-temperatures is photosystem-I, not photosystem-II. Planta 1994; 193:300 - 306
- Khorobrykh S, Mubarakshina M, Ivanov B. Photosystem I is not solely responsible for oxygen reduction in isolated thylakoids. Biochem Biophys Acat—Bioenergetics 2004; 1657:164 - 167
- Sundquist A, Fahey R. The function of gamma-glutamylcysteine and bis-gamma-glutamylcystine reductase in Halobacterium halobium. J Biol Chem 1989; 264:719