Abstract
Mitochondrial alternative oxidase (AOX), the unique respiratory terminal oxidase in plants, catalyzes the energy wasteful cyanide (CN)-resistant respiration and plays a role in optimizing photosynthesis. Recent studies from our group indicated that AOX plays a crucial role in chloroplast protection under extreme environments, such as high light (HL). Genetic data suggest that AOX is upregulated by light that was mediated by photoreceptors (phytochromes, phototropins and cryptochromes), and it also might have a particular role in relieving the overreduction of chloroplasts. Physiological analyses further suggest that AOX is essential for the dark-to-light transition, especially in de-etiolation course. In this mini-review, we highlight recent progresses in understanding the beneficial interaction between photosynthesis and mitochondria metabolism and discuss the possible role and mechanism of AOX in dissipation of excess reduced equivalents for chloroplasts under high light condition.
Introduction
For the last two decades, metabolic interaction between photosynthesizing chloroplasts and oxidative-respiring mitochondria has been intensively studied.Citation1,Citation2 In illuminated leaves, intracellular metabolism is dynamically modulated depending on environmental changes. Previous studies showed that excess light energy is harmful for plants and leads to disruption of the photosynthetic apparatus, especially the accumulation lots of reducing equivalents by the photochemical reaction in the thylakoid membrane of chloroplast under high light (HL) conditions.Citation3–Citation6 Depending on the developmental stage and/or environment, reducing equivalents generated in the form of NADPH are often in excess in the chloroplast stroma.Citation7 The accumulation of reducing equivalents in the stroma causes over-reduction of the photosynthetic electron transport chain and accelerates generation of ROS. Under such conditions, the function of chloroplasts and mitochondria is closely coordinated. Recently, metabolic interaction between chloroplasts and mitochondria has been intensively studied.Citation1 It has been suggested that AOX plays an essential role in preventing chloroplast over-reduction through the efficient dissipation of excess cellular reducing equivalents.Citation8,Citation9 These results may suggest that AOX would function as a sink for excess reducing equivalents generated in the chloroplasts, and this role of AOX has attracted much attention in terms of its interaction with photosynthesis. However, evidence for the role of AOX as a system for dissipation of excess reducing equivalents is still weak. Here, we reviewed and discussed the recent advances about the possible relationships between photosynthesis and mitochondrial metabolism and the light signaling involved in it.
AOX Gene Families and Physiological Functions in Plants
Mitochondria in higher plants have two ubiquinol-oxidizing pathways in the respiratory chain. One is the cytochrome pathway (CP), which is common in the respiratory chain in all aerobic organisms, and the other is the cyanide-insensitive alternative oxidase (AOX).Citation9,Citation10 The AOX branches from the cytochrome pathway at the level of the ubiquinone pool and couples the oxidation of ubiquinol to the four-electron reduction of oxygen to water. In angiosperms, two types of AOX genes exist: AOX1 genes have been found in all angiosperms examined to date, while AOX2 genes appear to be limited to dicot speces.Citation11,Citation12 In Arabidopsis, there are five AOX genes: AOX1a, AOX1b, AOX1c, AOX1d and AOX2.Citation13,Citation14
Accumulating evidence suggests that AOX may play a significant role in cell adaptation under different types of stress. Changes in AOX gene expression and AOX protein activity are induced in response to diverse biotic and abiotic stresses.Citation15,Citation16 As was suggested a long time ago and as has been shown by the results of many studies, AOX reduces the effects of oxidative stress by preventing accumulation of ROS in mitochondria. Moreover, ROS was regarded as a signaling molecule that can induce or upregulate AOX expression. However, there are multiple signaling pathways leading to AOX induction that can be either ROS-dependent or -independent.Citation17 Therefore, what the true factors are that affect AOX expression is largely dependent upon a given set of metabolic conditions. Recently, many works have demonstrated that in plants AOX can be used as a functional marker for efficient cell reprogramming under stress.Citation18–Citation20
The Possible Role of AOX in Balancing Photosynthesis and Respiration Metabolism Under High Light (HL)
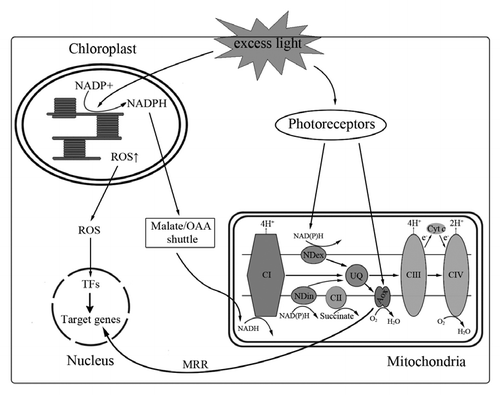
Light is a key regulator of gene expression in plants, altering the transcription of thousands of genes through direct (photo-receptor-mediated) or indirect (photosynthetic product-mediated) pathways.Citation21 However, the vast majority of detailed studies on light regulation have focused on photosynthesis-associated nuclear genes, and little is known about the effects of light on mitochondria and the respiratory chain. Recently, it has been demonstrated that the type II NAD(P)H dehydrogenase genes (nda1 and nadc1) are regulated by light in potato (Solanum tuberosum) and ArabidopsisCitation22,Citation23 and that the alternative oxidase genes are also light induced, not only monocots but also in dicots.Citation24–Citation27 The mode of signaling underlying light-regulated changes in mitochondria is unclear as of yet: how does the light signal reach the mitochondrion and how does the light signal modulate mitochondrial functions? Previous studies demonstrated that at least three pathways mediated the light-related cross-signaling between chloroplast and mitochondria ().
Under excess light conditions, AOX might serve to regenerate oxidised reductants (NAD+ and NADP+) and thereby prevent the inhibition of carbon metabolism under conditions of cytochrome (cyt) impairment.Citation9 Recently, many works have demonstrated that in plants AOX can be used to remove excess reducing power from the chloroplast.Citation25–Citation27 To prove this, AOX antisense and overexpressor lines were used to demonstrate that these reducing equivalents are transported from the chloroplast to the mitochondria where AOX serves to dissipate the excess reducing equivalents. Moreover, the oxaloacetate/malate shuttle was regarded as the most likely pathway for transporting those reducing equivalents from chloroplast to mitochondria, where they are taken up by the electron transport chain (ETC) and dissipated via mitochondrial non-phosphorylating pathways (including AOX).Citation2 Thus, AOX appears to contribute to the protection against photo-inhibition in plants.
In addition, controlling reactive oxygen species (ROS) generation may be another explanation for the role of AOX in alleviating photo-oxidative damage. As we all know, photosynthetic electron transport is known to be one of the main sources of ROS production in the photosynthesizing cell in light, and ROS generated in chloroplasts are involved in the cell metabolism and its regulation.Citation28,Citation29 Under light condition, ROS production increases with increasing light intensity.Citation30,Citation31 Earlier studies suggested that ROS can act as signal molecules in the cell and function as environmental sensors. Excess ROS imposes photo-oxidative damage to the photosynthetic apparatus.Citation2 AOX is thought to function as an antioxidant system and to suppress the generation of ROS.Citation9,Citation32 Thus, AOX would be essential for maintaining the photosynthetic electron transport chain in a more oxidized state, especially under stressful conditions. However, this idea may not necessarily be true in illuminated leaves, because they contain chloroplasts that are more susceptible to light energy and are the major sites of ROS generation in the light.Citation33 In the chloroplasts, ROS production could be avoided by several systems dissipating excess light energy, such as thermal dissipation of light energy by the conformational changes in photosystem II (PSII) and by the xanthophyll cycle, which is induced by PSI cyclic electron flow.Citation34 Therefore, it appears that upregulation of AOX expression and capacity under HL condition was affected directly or indirectly by ROS ().
In our study, more ROS and reducing equivalents accumulated in the Arabidopsis aox1a mutant than in wild type after HL treatment. In addition, all enzymes (NADP-MDH, NAD-MDH, citrate synthase (CS), NADP-ICDH and NAD-ME) involved in the malate/oxaloacetate shuttle were increased by the HL treatment and were always higher in the aox1a mutant after the HL treatment. Interestingly, ROS accumulation was so little in the aox1a mutant compared to the control that the role of AOX in relieving the over-reduction of chloroplasts may be more important than ROS scavenging in seedlings grown under HL conditions.Citation27
In addition to the indirect pathways of light-regulation of mitochondria described above, light could directly influence the respiratory electron transport chain via photoreceptor-mediated transcriptional control.Citation21,Citation35 Escobar et al.Citation21 demonstrated that alternative oxidase (AOX), uncoupling protein (UCP) and type II NAD(P)H dehydrogenase gene families were directly regulated by light in etiolated Arabidopsis seedlings. Feng et al.Citation26 showed that AOX pathway activation in the initial greening stage appears to be independent of photosynthetic metabolism and light would be a direct signal to induce the AOX pathway. In our work, we found that photoreceptors mediated light-induced AOX gene family expression in Arabidopsis. When Arabidopsis seedlings were illuminated with red (R) or blue (B) light, AOX1a expression could be doubled; while a clear decrease in transcript abundance was apparent in the phyA or phyB mutant and no increase could be found for the phyA,phyB double mutant, indicating that photoreceptors are a central component of the light signaling transduction pathway to AOX genes.Citation27 In addition, analysis of the light-responsive motifs in AOX1a promoter further improved the critical functions of photoreceptors in light signaling to AOX.Citation27
Previous work demonstrated that AOX plays an important role in dark-to-light transition, especially in the course of de-etiolation. Several studies revealed that the AOX pathway was involved in chloroplast carbon assimilation.Citation36,Citation37 Chlorophyll accumulation and CO2 fixation rate induced by light were largely delayed by SHAM (an AOX inhibitor) pretreatment and in the Ataox1a mutant,Citation26,Citation27 which is likely to be related to the inactivion or downregulation, respectively, of alternative pathways. These results imply that the AOX pathway may play a key role in chloroplast functions and that there might be a retrograde signaling from mitochondria to chloroplasts ().
Perspectives
Several studies on the modulation of photosynthesis and respiration have been made using light as one of the regulatory tools.Citation8 However, limited data are available, and the beneficial interaction between photosynthesis and mitochondria metabolism is still unclear. Under high light conditions, reductants are exported from the chloroplasts to the cytosol, probably via the NADP-MDH and Mal-OAA shuttle; and the respiratory chain is thought to dissipate excess reductants produced in the chloroplasts and AOX is thought to play an important role in relieving these reducing equivalents. Therefore, more research is needed to identify transporters functioning in the shuttle machinery from chloroplast to mitochondria in detail. Since ROS produced in the chloroplasts under excess light could induce AOX expression and capacity, further investigations are needed to examine the signal transduction pathways between ROS and AOX. Many more experiments are needed to characterize the nature and the role of the alternative pathway in the initial greening process of etiolated plants. What is the retrograde communication between chloroplast to mitochondria, and which process of mitochondrial retrograde regulation (MRR) occurs during the dark-to-light transition? Consequently, more attention should be paid to the effects of MRR on chlorophyll and chloroplast biosynthesis.
It is worth noting that mitochondrial uncoupling proteins (UCP) also play an important role in maintaining photosynthesis redox status.Citation38 Consequently, AOX probably works in conjunction with UCP under extreme environments, such as high light. Thus, further studies are needed to clarify the role and relationships of the two energy-dissipating respiratory proteins. Moreover, transgenic plants and novel experimental techniques are also needed to clarify the interaction between chloroplasts and mitochondria in the future.
Figures and Tables
Figure 1 Putative signaling pathways from light to organelles. Under excess light conditions, reducing equivalents (such as NADPH) generated in chloroplast which can be transported to mitochondria by Malate/OAA shuttle; Excess reducing equivalents can be dissipated by non-phosphorylating pathways, including NDin and AOX. Photoreceptors also mediate light-induced expression of NAD(P)H and AOX. In addition, alternative pathway may play an important role in the initial greening process of etiolated plants through mitochondrial MRR pathways. AOX, alternative oxidase; OAA, oxaloacetate; CI, Complex I; CII, Complex II; CIII, Complex III; CIV, Complex IV; Cyt c, cytochrome c oxidase; MRR, mitochondria retrograde regulation; NDex, external type II NAD(P)H dehydrogenase; NDin, internal type II NAD(P)H dehydrogenase; ROS, reactive oxygen species; TFs, target factors; UQ, ubiquinone.
Acknowledgements
This work was supported by the National Key Basic Research ‘973’ Program of China (2009CB118500), National Nature Science Foundation of China (30970214, 30800071, 31070210 and 910170004) and the Sichuan Nature Science Foundation (2010JQ0080).
References
- Noctor G, De Paepe R, Foyer CH. Mitochondrial redox biology and homeostasis in plants. Trends Plant Sci 2007; 12:125 - 134
- Noguchi K, Yoshida K. Interaction between photosynthesis and respiration in illuminated leaves. Mitochondrion 2008; 8:87 - 99
- Noguchi K, Go CS, Terashima I, Ueda S, Yoshinari T. Activities of the cyanide-resistant respiratory pathway in leaves of sun and shade species. Aust J Plant Physiol 2001; 28:27 - 35
- Noguchi K, Taylor NL, Millar AH, Lambers H, Day DA. Response of mitochondria to light intensity in the leaves of sun and shade species. Plant Cell Environ 2005; 28:760 - 771
- Yoshida K, Terashima I, Noguchi K. Upregulation of mitochondrial alternative oxidase concomitant with chloroplast overreduction by excess light. Plant Cell Physiol 2007; 48:606 - 614
- Yoshida K, Watanabe C, Kato Y, Sakamoto W, Noguchi K. Influence of chloroplastic photo-oxidative stress on mitochondrial alternative oxidase capacity and respiratory properties: a case study with Arabidopsis yellow variegated 2. Plant Cell Physiol 2008; 49:592 - 603
- Allen JF. Photosynthesis of ATP-electrons, proton pumps, rotors and poise. Cell 2002; 110:273 - 276
- Raghavendra AS, Padmasree K. Beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Trends Plant Sci 2003; 8:546 - 553
- McDonald AE. Alternative oxidase: an inter-kingdom perspective on the function and regulation of this broadly distributed ‘cyanide-resistant’ terminal oxidase. Funct Plant Biol 2008; 35:535 - 552
- Polidoros AN, Mylona PV, Arnholdt-Schmitt B. Aox gene structure, transcript variation and expression in plants. Physiol Plant 2009; 137:342 - 353
- Considine MJ, Holtzapffel RC, Day DA, Whelan J, Millar AH. Molecular distinction between alternative oxidase from monocots and dicots. Plant Physiol 2002; 129:949 - 953
- The Arabidopsis Genome Initiative, Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 2000; 408:796 - 815
- Clifton R, Millar AH, Whelan J. Alternative oxidases in Arabidopsis: A comparative analysis of differential expression in the gene family provides new insights into function of non-phosphorylating bypasses. Biochim Biophys Acta 2006; 1757:730 - 741
- Saisho D, Nambara E, Naito S, Tsutsumi N, Hirai A, Nakazono M. Characterization of the gene family for alternative oxidase from Arabidopsis thaliana. Plant Mol Biol 1997; 35:585 - 596
- Van Aken O, Giraud E, Clifton R, Whelan J. Alternative oxidase: a target and regulator of stress responses. Physiol Plant 2009; 137:354 - 361
- Vanlerberghe GC, Cvetkovska M, Wang J. Is the maintenance of homeostatic mitochondrial signaling during stress a physiological role for alternative oxidase?. Physiol Plant 2009; 137:392 - 406
- Gray GR, Villarimo AR, Whitehead CL, McIntoch L. Transgenic tobacco (Nicotiana tabacum L.) plants with increased expression levels of mitochondrial NADP+-dependent isocitrate dehydrogenase: evidence implicating this enzyme in the redox activation of the altervative oxidase. Plant Cell Physiol 2004; 45:1413 - 1425
- Umbach AL, Fiorani F, Siedow JN. Characterization of transformed Arabidopsis with altered alternative oxidase levels and analysis of effects on reactive oxygen species in tissue. Plant Physiol 2005; 139:1806 - 1820
- Fiorani F, Umbach AL, Siedow JN. The alternative oxidase of plant mitochondria is involved in the acclimation of shoot growth at low temperature. A study of Arabidopsis AOX1a transgenic plants. Plant Physiol 2005; 139:1795 - 1805
- Arnholdt-Schmitt B, Costa JH, de Melo DF. AOX—a functional marker for efficient cell reprogramming under stress?. Trends Plant Sci 2006; 11:281 - 287
- Escobar MA, Franklin KA, Svensson AS, Salter MG, Whitelam GC, Rasmusson AG. Light regulation of the Arabidopsis respiratory chain. Multiple discrete photo-receptor responses contribute to induction of type II NAD(P)H dehydrogenase genes. Plant Physiol 2004; 136:2710 - 2721
- Svensson AS, Rasmusson AG. Light-dependent gene expression for proteins in the respiratory chain of potato leaves. Plant J 2001; 28:73 - 82
- Michalecka AM, Svensson AS, Johansson FI, Agius SC, Johanson U, Brennicke A, Rasmusson AG. Arabidopsis genes encoding mitochondrial type II NAD(P)H dehydrogenases have different evolutionary origin and show distinct responses to light. Plant Physiol 2003; 133:642 - 652
- van Lis R, Atteia A. Control of mitochondrial function via photosynthetic redox signals. Photosynth Res 2004; 79:133 - 148
- Yoshida K, Terashima I, Noguchi K. Distinct roles of the cytochrome pathway and alternative oxidase in leaf photosynthesis. Plant Cell Physiol 2006; 47:22 - 31
- Feng HQ, Li HY, Li X, Duan JG, Liang HG, Zhi DJ, Ma J. The flexible interrelation between AOX respiratory pathway and photosynthesis in rice leaves. Plant Physiol Biochem 2007; 45:228 - 235
- Zhang DW, Xu F, Zhang ZW, Chen YE, Du JB, Jia SD, et al. Effects of light on cyanide-resistant respiration and alternative oxidase function in Arabidopsis seedlings. Plant Cell Environ 2010; 33:2121 - 2131
- Mubarakshina MM, Ivanov BN. The production and scavenging of reactive oxygen species in the plastoquinone pool of chloroplast thylakoid membranes. Physiol Plant 2010; 140:103 - 110
- Foyer CH, Noctor G. Redox regulation in photosynthetic organisms: signaling, acclimation and practical implications. Antioxid Redox Signal 2009; 11:861 - 905
- Mubarakshina M, Khorobrykh S, Ivanov B. Oxygen reduction in chloroplast thylakoids results in production of hydrogen peroxide inside the membrane. Biochim Biophys Acta 2006; 1757:1496 - 1503
- Mubarakshina MM, Khorobrykh SA, Kozuleva MA, Ivanov BN. Intramembrane formation of hydrogen peroxide during oxygen reduction in thylakoids of higher plants. Dokl Biokhim Biofiz 2006; 408:113 - 116
- Maxwell DP, Wang Y, McIntosh L. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci USA 1999; 96:8271 - 8276
- Foyer CH, Noctor G. Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol Plant 2003; 119:355 - 364
- Shikanai T. Cyclic electron transport around photosystem I: genetic approaches. Annu Rev Plant Biol 2007; 58:199 - 217
- Liscum E, Hodgson DW, Campbell TJ. Blue light signaling through the cryptochromes and phototropins. Plant Physiol 2003; 133:1429 - 1436
- Bartoli CG, Gomez F, Gergoff G, Guiamet JJ, Puntarulo S. Upregulation of the mitochondrial alternative oxidase pathway enhances photosynthetic electron transport under drought conditions. J Exp Bot 2005; 56:1269 - 1276
- Padmasree K, Raghavendra AS. Response of photosynthetic carbon assimilation in mesophyll protoplasts to restriction on mitochondrial oxidative metabolism: Metabolites related to the redox status and sucrose biosynthesis. Photosynth Res 1999; 62:231 - 239
- Sweetlove LJ, Lytovchenko A, Morgan M, Nunes-Nesi A, Taylor NL, Baxter CJ, et al. Mitochondrial uncoupling protein is required for efficient photosynthesis. Proc Natl Acad Sci USA 2006; 103:19587 - 19592