Abstract
Cellulose biosynthesis inhibitors, such as dichlobenil (DCB), have become a valuable tool for the analysis of structural and compositional plasticity of plant cell walls. By stepwise increasing the concentration of DCB in the culture medium, we obtained maize cells able to cope with DCB through the acquisition of a modified cell wall in which cellulose was partially replaced by a more extensive network of feruloylated arabinoxylans. Recently we demonstrated that the expression of several Cellulose Synthase and phenylpropanoid-related genes is altered in DCB-habituated cells. In addition, by using a proteomic approach we identified several proteins induced or repressed in DCB-habituated cells. After a more in-depth analysis, some new proteins induced (two inhibitors TAXI-IV, an α-1,4-glucan-protein synthase, and a pectinesterase inhibitor) or repressed (a chaperonin 60, a fructokinase-1 and a spermidine synthase 1) were identified, and their possible role in the context of DCB-habituation is discussed.
The primary cell wall of grasses and related commelinoid monocots (Type II) is composed of a framework of cellulose microfibrils embedded in a matrix of hemicellulosic polysaccharides (arabinoxylans, xyloglucan and mixed-linked glucans) and smaller amounts of pectins and glycoproteins.Citation1 Nowadays, there is increasing evidence to suggest that plant cells are capable of monitoring the functional integrity of the primary cell wall.Citation2–Citation4
Owing to the key role of cellulose in the cell wall structure, cellulose biosynthesis inhibitors (CBIs) have become a valuable tool for the analysis of cell wall structure and biogenesis.Citation5,Citation6 Cell cultures of several species have been habituated to grow in the presence of several CBIs by incremental exposure over many culturing cycles.Citation7–Citation9 We obtained maize cell lines habituated to lethal concentrations of dichlobenil (2,6-dichlorobenzonitrile, DCB).Citation10 These cell cultures were enabled to grow through the acquisition of a modified type II cell wall in which cellulose was reduced and partially replaced by a more extensive network of arabinoxylans. Moreover, cell wall hydroxycinnamates, the units linking arabinoxylans, experienced quantitative and qualitative changes.Citation11 At a molecular level, the expression of several Cellulose Synthase and phenylpropanoid-related genes was altered. In addition, by using a proteomic approach, other proteins related to different metabolisms such as carbon, nitrogen and ethylene or detoxification mechanisms were found to be altered.Citation12
After a more in-depth analysis of proteomic results, some new proteins with putative relevance for the habituation process were identified. Here, we report those proteins which seem to contribute to the maintenance of cell wall integrity/cell survival following cellulose biosynthesis inhibition by DCB ( and ).
Two maize xylanase inhibitors belonging to TAXI-IV type (Triticum aestivum xylanase inhibitor IV) were induced by DCB-habituation, in addition to the putative xylanase inhibitor previously reported.Citation12 Previous results showed that the architecture of type II cell walls is able to compensate for deficiencies in cellulose content by producing a more extensive network of arabinoxylans. In fact, it has been demonstrated that arabinoxylans from DCB-habituated cell walls were characterized by a marked increase in their relative molecular mass when compared with those obtained from control cells.Citation10 In the context of DCB-habituation it is tempting to consider that xylanase inhibition plays a role in preventing the depolymerisation of endogenous arabinoxylan.
So far, the relationship between DCB-habituation and TAXI proteins remains unexplained. In a recent paper, it was demonstrated that TAXI-I gene expression is specifically induced by the stress phytohormone methyl jasmonate.Citation13 Therefore, it would be plausible to consider TAXI induction as a general response to a putative DCB-induced stress. However, TAXI type proteins specifically inhibit bacterial and fungal endo-β-1,4-xylanases (G11 group) and therefore it has been suggested that they participate in plant defence (it is the socalled plant defense hypothesis).Citation14,Citation15 As TAXI type proteins do not seem to be active against plant endoxylanases (to date, all of them identified belonging to G10 group), a role for these proteins in the remodelling of cell wall arabinoxylans is unlikely.Citation14–Citation16 Nevertheless, as the inhibition specificity for TAXI-IV proteins has not been reported yet,Citation14 further research will be needed to discard this possibility.
In addition, other cell wall related proteins were induced in DCB-habituated cells, including a pectinesterase inhibitor, which inhibits the action of pectin methylesterases through the formation of a non-covalent complex. Pectin methylesterases have a prominent role in pectin metabolism since they can promote both pectin Ca2+ binding and pectin susceptibility to specific-degrading enzymes.Citation17 Although pectins are not major components of type II cell walls, the induction of a pectin methylesterase inhibitor in DCB-habituated cells raises the question of whether this kind of enzyme plays a significant role in the modification of the maize cell wall architecture in this process.
An α-1,4-glucan-protein synthase (UDP forming; UPTG), a self glycosylation protein, has also been found to be induced in DCB-habituated cells, and it has already been suggested that this has a role in the synthesis of hemicellulosic polysaccharides.Citation18 Interestingly, a positive correlation between UPTG transcript levels and the active synthesis of cell wall components was found in potato.Citation19 Moreover, in accordance with the high glycosylation rates of UPTG for xylose and galactose, it has been proposed a role for this protein in xyloglucan biosynthesis,Citation19 this being the main hemicellulosic polysaccharide in type I cell walls. In our case, it will be necessary to address the possible link between increased UPTG activity and the active synthesis of arabinoxylan during DCB-habituation, as it has been demonstrated that habituated cells replace cellulose by increasing the incorporation of arabinoxylans into the cell wall,Citation10 and these play a similar role in type II cell walls to that played by xyloglucan in type I.
It has been shown that hexoses may function as stress indicators when cell wall integrity is impaired.Citation2 In line with this, we have reported three proteins which were altered during DCB-habituation, and which are involved in hexoses metabolism. Here we show that fructokinase-1, which may be directly or indirectly involved in a sugar-sensing pathway,Citation20,Citation21 was reduced by the habituation. Based on these results, future research is needed to clarify the exact role of hexoses as cell wall stress indicators.
Another interesting scenario would indicate fructokinase-1 as regulating the sucrose metabolism of habituated cells. As fructokinase phosphorylates fructose to fructose-6P, reduced fructokinase activity would inhibit sucrose synthase activity by a fructose accumulation feedback mechanism.Citation22 Sucrose synthase has a central role in directing carbon flow to sugar metabolism and cell wall synthesis. Therefore, DCB-habituated cells might be expected to have reduced sink strength.
Spermidine synthase 1, involved in the synthesis of the polyamine spermidine, was repressed in DCB-habituated cells. Although numerous unanswered questions remain regarding polyamines in plants, spermidine is essential, and one of the reasons for this may lay in the fact that spermidine serves as a substrate for post-translational modification of the eukaryotic translation initiation factor 5A, which is essential in all eukaryotic cells.Citation23 Interestingly, this has been shown to increase in DCB-habituated cells.Citation12 In this case, it remains to be confirmed whether the increase shown in translation initiation factor 5A is related to the decrease in spermidine.
It has been demonstrated that under stress conditions, the requirement for chaperone function increases, in order to prevent and reverse incorrect interactions of proteins.Citation24 However, chaperonin 60, another stress-related protein was again found to be repressed in DCB-habituated cells. The repression of these stress-related proteins contrasts with the fact that other stress-related proteins (heat shock protein 70 and peroxidase 52 precursor) were previously reported as having been induced during the habituation to DCB,Citation12 demonstrating the precise strategy followed by cells habituated to grow in the presence of high DCB concentrations.
In conclusion and on the basis of these and previous results,Citation10–Citation12 both the modified cell wall architecture and a very precise control of stress responses seem to be responsible for the capacity displayed by DCB-habituated cells to survive in the presence of lethal concentrations of a cellulose biosynthesis inhibitor. These results prompt us to continue investigating the upstream signals controlling all these downstream processes.
Figures and Tables
Figure 1 2-D resolution of the maize cultured cells proteins. Comparison of the total proteins of non-habituated (A) and DC B-habituated (B) maize cells. Numbers on the left indicate the positions of the relative molecular mass standards (X1,000 Da), and the isoelectric point (pI) is given at the top. Gels were loaded with 200 µg proteins and visualized by silver staining. The open white circles correspond to the selected spots analyzed by MALDI-TOF/MS or by LC-nanoESI-Q-TOF-MS/MS, which are listed in .
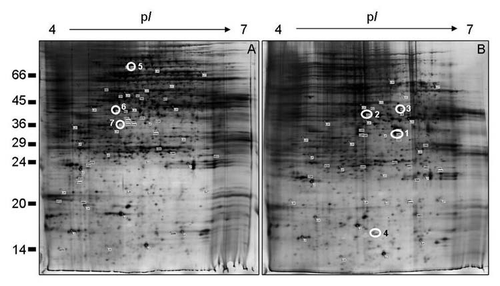
Table 1 Identification of proteins by MALDI-TOF/MS or by LC-nanoESI-Q-TOF-MS/MS after spot excision of 2-D gels (see ), with an indication of their theoretical and experimental isoelectric point (pI) and molecular mass (MM (Da)
Acknowledgements
This work received financial support from the Spanish Ministry of Science and Innovation (CGL2008-02470/BOS and AGL2008-05157) and the Junta de Castilla y León (LE044A10-2). DCR was financed by the Spanish Ministry of Science and Innovation (“I3” Program). We are indebted to S. Irar for his technical support as head of the proteomic service at CRAG.
Addendum to:
References
- Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants. Consistency of molecular structure with the physical properties of the walls during growth. Plant J 1993; 3:1 - 30
- Hamann T, Bennett M, Mansfield J, Somerville C. Identification of cell-wall stress as a hexose-dependent and osmosensitive regulator of plant responses. Plant J 2009; 57:1015 - 1026
- Ringli C. Monitoring the outside: Cell wall-sensing mechanisms. Plant Physiol 2010; 153:1445 - 1452
- Seifert GJ, Blaukopf C. Irritable walls: The plant extracellular matrix and signaling. Plant Physiol 2010; 153:467 - 478
- Vaughn KC. Böger P, Wakabayashi K, Hirai K. Cellulose biosynthesis inhibitor herbicides. Herbicide Classes in Development 2002; Berlin Springer-Verlag 139 - 150
- Acebes JL, Encina A, García-Angulo P, Alonso-Simón A, Mélida H, Álvarez JM. Lejeune A, Deprez T. Cellulose biosynthesis inhibitors: Their uses as potential herbicides and as tools in cellulose and cell wall structural plasticity research. Cellulose: Structure and Properties, Derivatives and Industrial Uses 2010; New York Nova Publishers 39 - 73
- Shedletzky E, Shmuel M, Trainin T, Kalman S, Delmer D. Cell-wall structure in cells adapted to growth on the cellulose-synthesis inhibitor 2,6-dichlorobenzonitrile—a comparison between two dicotyledonous plants and a gramineous monocot. Plant Physiol 1992; 100:120 - 130
- Encina A, Moral RM, Acebes JL, Álvarez J. Characterization of cell walls in bean (Phaseolus vulgaris L.) callus cultures tolerant to dichlobenil. Plant Sci 2001; 160:331 - 339
- Manfield IW, Orfila C, McCartney L, Harholt J, Bernal AJ, Scheller HV, et al. Novel cell wall architecture of isoxaben habituated Arabidopsis suspension cultured cells: global transcript profiling and cellular analysis. Plant J 2004; 40:260 - 275
- Mélida H, García-Angulo P, Alonso-Simón A, Encina A, Álvarez J, Acebes JL. Novel type II cell wall architecture in dichlobenil-habituated maize calluses. Planta 2009; 229:617 - 631
- Mélida H, García-Angulo P, Alonso-Simón A, Álvarez JM, Acebes JL, Encina A. The phenolic profile of maize primary cell wall changes in cellulose-deficient cell cultures. Phytochemistry 2010; 71:1684 - 1689
- Mélida H, Encina A, Álvarez J, Acebes JL, Caparrós-Ruiz D. Unraveling the biochemical and molecular networks involved in maize cell habituation to the cellulose biosynthesis inhibitor dichlobenil. Mol Plant 2010; 3:842 - 853
- Weng X, Huang Y, Gao H, Sun J. Characterization of a xylanase inhibitor TAXI-I from wheat. Biol Plant 2010; 54:154 - 158
- Dornez E, Croes E, Gebruers K, De Coninck B, Cammue BPA, Delcour JA, Courtin CM. Accumulated evidence substantiates a role for three classes of wheat xylanase inhibitors in plant defense. Crit Rev Plant Sci 2010; 29:244 - 264
- Gebruers K, Brijs K, Courtin CM, Fierens K, Goesaert H, Rabijns A, et al. Properties of TAXI-type endoxylanase inhibitors. Biochim Biophys Acta 2004; 1696:213 - 221
- Igawa T, Ochiai-Fukuda T, Takahashi-Ando N, Ohsato S, Shibata T, Yamaguchi I, Kimura M. New TAXI-type xylanase inhibitor genes are inducible by pathogens and wounding in hexaploid wheat. Plant Cell Physiol 2004; 45:1347 - 1360
- Pelloux J, Rustérucci C, Mellerowicz EJ. New insights into pectin methylesterase structure and function. Trends Plant Sci 2007; 12:267 - 277
- Dhugga KS, Ulvskov P, Gallagher SR, Ray PM. Plant polypeptides reversibly glycosylated by UDPglucose—possible components of Golgi beta-glucan synthase in pea cells. J Biol Chem 1991; 266:21977 - 21984
- Wald FA, Kissen R, du Jardin P, Moreno S. Characterization of UDP-glucose: Protein transglucosylase genes from potato. Plant Mol Biol 2003; 52:705 - 714
- Pego JV, Smeekens SCM. Plant fructokinases: A sweet family get-together. Trends Plant Sci 2000; 5:531 - 536
- Zhang SR, Nichols SE, Dong JG. Cloning and characterization of two fructokinases from maize. Plant Sci 2003; 165:1051 - 1058
- Schaffer AA, Petreikov M. Inhibition of fructokinase and sucrose synthase by cytosolic levels of fructose in young tomato fruit undergoing transient starch synthesis. Physiol Plant 1997; 101:800 - 806
- Takahashi T, Kakehi J. Polyamines: Ubiquitous polycations with unique roles in growth and stress responses. Ann Bot 2010; 105:1 - 6
- Li K, Xu C, Zhang K, Yang A, Zhang J. Proteomic analysis of roots growth and metabolic changes under phosphorus deficit in maize (Zea mays L.) plants. Proteomics 2007; 7:1501 - 1512