Abstract
Post-transcriptional gene regulation by microRNAs (miRNAs) and RNA-binding proteins (RBPs) is central to many biological functions. Aberrant gene expression patterns underlie many metabolic diseases that represent major public health concerns and formidable therapeutic challenges. Several studies have established a number of post-transcriptional regulators implicated in metabolic diseases such as diabetes and obesity. In addition, emerging knowledge of metabolically active and insulin-sensitive organs, such as the pancreas, liver, muscle and adipose compartment, is rapidly expanding the panel of potential therapeutic targets for the treatment of metabolic diseases. Here, we review our current understanding of miRNAs and RBPs that affect glucose and lipid homeostasis, and their roles in normal physiology and metabolic disorders, especially type 2 diabetes and obesity.
Introduction
Type 2 diabetes mellitus (T2DM) is a metabolic disease characterized by insulin resistance in insulin-sensitive tissues including white adipose tissue, skeletal muscle and liver as well as impaired insulin secretion from pancreatic β cells, resulting in hyperglycemia.Citation1,Citation2 Insulin resistance is defined as a state in which insulin becomes less effective at lowering blood glucose and occurs when increasing amounts of insulin in circulation are needed to maintain glucose homeostasis.Citation3 It commonly leads to impaired glucose and lipid metabolism, which are major risk factors for development of metabolic disease.Citation4 It is important to characterize the molecular mechanisms of insulin resistance and subsequent pancreatic β-cell failure in order to develop approaches to prevent metabolic diseases. Despite considerable gains in our understanding of causes and risk factors for metabolic disease, the molecular mechanisms underlying insulin resistance and the pathogenesis of other metabolic diseases are unclear. Recent studies have extended this notion to posttranscriptional gene regulation.
Acute and prompt changes in mRNA levels are critical for altering the level of proteins in response to various metabolic stimuli and maintain cellular homeostasis. At the post-transcriptional level, gene regulation is governed by two major types of trans-binding factors: microRNAs (miRNAs) and RNA binding proteins (RBPs). miRNAs are a large group of small (19–23-nt-long) non-coding RNAs that critically influence gene expression by forming incomplete base pairing with target mRNAs.Citation5,Citation6 miRNAs are involved in diverse biological progresses, such as differentiation and development, and their dysregulation has been linked to many diseases.Citation7 Generally, miRNAs repress protein synthesis by enhancing mRNA decay or inhibiting translation through their interaction with cis-elements of target mRNAs, although, some miRNAs were reported to activate mRNA translation.Citation8-Citation10 RBPs (especially, turnover- and translation-regulatory RBPs, TTR-RBPs) regulate mRNA stability and translation rate by interacting with target mRNAs via different RNA interaction motifs.Citation11 RBPs are varied in both structure and complexity, and perform diverse functions affecting all aspects of RNA metabolism (reviewed in ref. Citation12). miRNAs and RBPs generally interact with the 3′-untranslated region (UTR) of target mRNAs and modulate mRNA stability and translation. However, increasing evidences indicate that those trans-binding factors also interact with the coding region and with the 5′-UTR.Citation13,Citation14
Recently, significant progress has been made in understanding the cellular roles of post-transcriptional regulators in metabolically active and insulin-sensitive tissues. Their influence on metabolism and cellular homeostasis in vivo needs to be further characterized systematically. Here, we review the most recent findings of miRNAs and RBPs involved in the maintenance of glucose and lipid homeostasis, and their implication in normal physiology and in metabolic disorders, especially T2DM and obesity.
Glucose homeostasis
In mammals, glucose homeostasis is determined by the minute-to-minute control of the amount of circulating insulin levels and the number of insulin-secreting β- cells in islets. An inadequate expansion of β-cell mass or failure of the existing β-cell mass to compensate for increased insulin demand, associated with eventual loss of β- cells due to apoptosis, are hallmarks of T2DM. These prominent features of T2DM result from a defect in insulin action mediated by the insulin receptor (IR).Citation15-Citation20 Insulin binding to IR activates a intracellular signaling pathway including the IR, insulin receptor substrate (IRS), phosphoinositide 3-kinase (PI3K), phosphoinositide-dependent kinase 1 (PDK1), and the protein kinase AKT, and thereby regulates not only glucose and lipid metabolism but also cellular proliferation and apoptosis.Citation15-Citation23 Indeed, studies of animal models with targeted mutations in genes that encode mediators of insulin signaling have revealed that insulin signaling plays a central role in metabolic actions in insulin-sensitive tissues and contributes to the pathogenesis of T2DM.Citation21-Citation23 Dysregulation at any step of this fine tuning is responsible for insulin resistance and impaired glucose homeostasis, resulting from diminished glucose uptake and utilization in insulin-sensitive tissues.
miR-375 in glucose homeostasis
The link between miRNA and diabetes was brought to light with the discovery of miR-375, which is one of the most abundant miRNAs present in pancreatic islets and is a major mediator of β-cell function and hence glucose homeostasis.Citation24,Citation25 The expression of miR-375 is under the control of PDX-1, a major transcriptional regulator of β-cell function and mass.Citation26 The first study of miR-375 in insulin-secreting MIN6 and primary β-cells revealed that miR-375 negatively regulates glucose-stimulated insulin secretion with no effect on either ATP production or intracellular Ca2+ levels.Citation24 It influences a late step of insulin exocytosis through a reduction in the expression of myotrophin, a protein involved in insulin granule fusion. Subsequently, miR-375 was shown to be involved in glucose-mediated actions on insulin gene expression through the regulation of PDK1, a key protein in PI3K signaling in pancreatic β- cells.Citation25 Moreover, miR-375 expression is regulated by glucoseCitation27 and its levels in pancreatic islets are aberrant in obese ob/ob mice,Citation28 a model of T2DM. These findings suggest that miR-375 is involved in the regulation of glucose responsiveness in β-cells and therefore it has implication in the pathogenesis of T2DM. Indeed, mice lacking miR-375 exhibited fasting and non-fasting hyperglycemia and glucose intolerance; this phenotype was a result of upregulated gluconeogenesis and increased hepatic glucose output due to the higher total pancreatic α-cell compartment and resultant hyperglucagonemia.Citation28 In contrast, pancreatic β-cell mass is decreased in these mice as a result of impaired proliferation. In agreement with these observations, genetic deletion of miR-375 in obese ob/ob mice led to a severe diabetic state, because of a profoundly diminished proliferative capacity of β cells. Thus, miR-375 is at present the best-studied miRNA implicated in β-cell function as well as glucose homeostasis.
Lin28/ let-7a in glucose homeostasis
The tumor-suppressor miRNA let-7 negatively regulates the expression of oncogenes and cell cycle regulators,Citation29-Citation32 while, the RNA-binding proteins Lin28a and Lin28b are activated in many cancers to selectively and post-transcriptionally inhibit let-7 biogenesis.Citation32-Citation35 A recent study by Zhu and colleagues uncovered a connection between lin28/let-7 signaling and glucose metabolism.Citation36 Both Lin28a and Lin28b transgenic mice have improved insulin sensitivity and glucose homeostasis, and are resistant to obesity, whereas muscle-specific Lin28a knockout mice display insulin resistance and glucose intolerance. These effects are mediated by an increase in insulin-PI3K-mTOR signaling due in part to the let-7-mediated repression of multiple targets in the pathway, including insulin-like growth factor 1 receptor (IGF1R), IR, IRS2 and AKT2. Moreover, overexpression of let-7 caused impaired insulin sensitivity and glucose homeostasis, suggesting that Lin28a/b exerts effects on glucose metabolism at least in part by suppressing let-7 levels. The connection between lin28/let-7 signaling and glucose homeostasis is further supported by the observation that many genes associated with T2DM and control of fasting glucose in human are known or predicted let-7 targets.Citation36 Thus, although the function and regulation of lin28 and let-7 remain to be studied in more detail in animal models, the lin28/let-7 signaling axis may represent a valuable therapeutic target in obesity and diabetes.
microRNAs in insulin sensitivity
It is clear that various miRNAs contribute to the development of insulin resistance due to alteration of gene expression in insulin-sensitive tissues (reviewed in ref. Citation37). Recently, several microRNAs have been found to directly regulate insulin sensitivity.Citation38,Citation39 Trajkovski et al. found that miR-103 and miR-107 negatively regulate insulin sensitivity: both were upregulated in both genetic and diet-induced obese mice.Citation38 The expression of miR-103/107 in either liver or white adipose tissue disrupted glucose homeostasis and, conversely, global miR-103/107 silencing resulted in improved glucose homeostasis and insulin sensitivity, implicating these miRNAs as novel therapeutic targets for the treatment of diabetes. The same investigation also found that these effects are mediated by targeting caveolin-1, resulting in a reduction in the abundance of IRs in caveolae-enriched plasma membrane microdomains and reducing downstream insulin signaling.
The upregulation of miR-143 was also confirmed in the liver of both genetic- and diet-induced obese mice.Citation39 This observation is consistent with other reports showing that miR-143 is dysregulated in tissues of ob/ob,Citation40 diet-induced obeseCitation41,Citation42 and diabetic miceCitation43 as well as in human patients.Citation44 Gain- and loss-of-function studies also confirmed miR-143 as a negative regulator of insulin sensitivity and glucose homeostasis, resulting from impaired insulin signaling via AKT.Citation39
microRNAs in insulin biosynthesis
A review of RBPs and microRNAs that target the mRNAs encoding insulin and its receptors was lately publishedCitation45 and additional RBPs and microRNAs have since been reported. miR-24, miR-26, miR-148 and miR-182 were found to be involved in increasing insulin promoter activity and insulin mRNA levels, while miRNA-dependent regulation of insulin expression was associated with upregulation of transcriptional repressors such as Bhlhe22 (basic helix-loop-helix family member 22) and Sox6 (Sry-related HMG box 6).Citation46
RBPs in glucose homeostasis
Several RBPs were reported to be involved in insulin biosynthesis and glucose homeostasis. Kulkarni and coworkers reported protein-disulfide isomerase (PDI) and poly(A)-binding protein as trans-acting factors that bind to the 5′ UTR of insulin mRNA and mediate glucose-stimulated translation of insulin mRNA in pancreatic islets.Citation47 ARE/poly(U)-binding factor 1 (AUF1), the best-characterized RBP that controls mRNA stability and translation by associating with AU- or U-rich sequence elements (ARE) frequently found within the 3′-UTR, was found to be involved in the cytotoxic effects of proinflammatory cytokines on pancreatic β cells.Citation48 Exposure of pancreatic β cells to cytokines caused translocalization of AUF1 to the cytoplasm. Overexpression of AUF1 induced β-cell death due to a decrease in the expression of the anti-apoptotic proteins, whereas knockdown of AUF1 restored the levels of the anti-apoptotic proteins, attenuated the activation of the nuclear factor-κB pathway, and protect the β cells from cytokine-induced apoptosis.
Studies by Welsh group have shown that glucose-stimulated binding of the polypyrimidine tract-binding protein (PTB), also known as heterogeneous nuclear ribonucleoprotein I (hnRNP I), to the 3′-UTR of the preproinsulin mRNA, stabilizes the messengers, resulting in the glucose-stimulated increase in preproinsulin mRNA.Citation49,Citation50 Additionally, PTB plays a role in insulin secretion by regulating the biogenesis of insulin secretory granules.Citation51 Glucose stimulation of pancreatic β cells induced the cytosolic localization of PTB, thereby participating in the stabilization of mRNAs encoding proteins on secretory granules. Conversely, knockdown of PTB expression led to the depletion of secretory granules as well as decreased levels of intracellular and secreted insulin.
Recently, we identified HuD/ELAVL4 (human antigen D/ embryonic lethal abnormal vision-like 4) as an RBP that binds to the insulin mRNA and controls its translation.Citation52 Similar to two other Hu/ELAV family members (HuB and HuC), HuD was believed to be expressed specifically in neurons.Citation53,Citation54 However, we found that HuD is also present in pancreatic β cells, where its levels are controlled by the IR pathway.Citation52 In β cells, HuD binding to the Ins2 5′UTR repressed Ins2 mRNA translation and decreased insulin production. Accordingly, HuD-null mice expressed higher levels of insulin in β cells, while HuD-transgenic mice expressed lower insulin levels in β cells. Importantly, HuD-transgenic mice displayed impaired glucose homeostasis because of decreased insulin secretion, thus defining HuD as a pivotal regulator of insulin translation.
Lipid homeostasis
Cholesterol is a major component of cell membranes and is synthesized endogenously in a highly regulated enzymatic reaction. Cellular lipid levels are tightly regulated by critical factors; peroxisome proliferator-activated receptors (PPARs), sterol regulatory element-binding proteins (SREBPs) and liver X receptor (LXR) (reviewed in refs. Citation55-Citation57). To maintain cholesterol homeostasis, excess cholesterol is exported from cells to apoA1 acceptors via the adenosine triphosphate-binding cassette (ABC) transporters, ABCA1 and ABCG1.Citation58 Dysregulation of lipid homeostasis is associated with the development of several diseases such as cancers and atherosclerosis.Citation59-Citation61
miR-122 in cholesterol metabolism
Several groups have assessed the role of miR-122 in lipid metabolism by using antisense methods to inhibit the expression of miR-122.Citation62-Citation64 miR-122 is highly conserved and the most abundantly expressed miRNA which regulate cholesterol and lipid homeostasis in the liver.Citation62-Citation64 Krütfeldt and colleagues showed that inhibition of miR-122 using 2′-O-methoxyethyl (2′-O-MOE) phosphorothioate antisense oligonucleotide resulted in complete loss of miR-122 in the liver leading to the upregulation of a number of genes involved in cholesterol biosynthesis.Citation62 Although they observed an increase of several genes that contain putative binding sites for miR-122, it needs to be determined which direct target mRNAs are responsible to miR-122-mediated cholesterol biosynthesis. Esau et al. also reported that miR-122 knockdown reduced circulating cholesterol level and fatty acid synthesis, while it elevated liver fatty acid β-oxidation, indicating that inhibition of miR-122 reduces hepatic cholesterol accumulation and improves liver steatosis in diet-induced obesity.Citation63 Similarly, locked-nucleic acid (LNA)-antagonism of miR-122 in mice increased the expression of predicted target genes involved in lipid biogenesis and affected cholesterol metabolism without liver toxicity,Citation64 suggesting that inhibition of miR-122 has a feasible therapeutic target .
miR-33 in cholesterol metabolism
SREBPs are helix-loop-helix-leucine zipper transcription factors that play critical roles in cholesterol biosynthesis, while miR-33a and miR-33b, encoded within the introns of the srebp-2 and srebp-1 genes, respectively, are highly conserved and ubiquitously expressed miRNAs.Citation65 Recently, it was reported that miR-33a/b are co-regulated with their host genes under metabolic stimuli and they were identified as critical regulators of cholesterol homeostasis by three different groups.Citation65-Citation68 In mouse and human, miR-33 inhibited the expression of the ABCA1, thereby inhibiting cholesterol trafficking. Overexpression of miR-33 in macrophages and hepatocytes decreased cholesterol efflux to apoA1, while inhibition of miR-33 resulted in increased ABCA expression and cholesterol efflux. In mouse macrophages, miR-33 also regulated ABCG1, which mobilized cellular free cholesterol to high density lipoprotein (HDL) particles.Citation65,Citation66 In human macrophages and hepatocytes, miR-33 regulated the expression of the protein NPC1 (Neimann Pick C1), which acts in concert with ABCA1 to cause efflux of cholesterol to apoA1, suggesting that miR-33 suppresses another component of the cholesterol export pathway in humans.Citation66 It was further demonstrated that miR-33 also affects β-oxidation of fatty acids by targeting CPT1a, CROT and HADHB, which are critical enzymes in the β-oxidation pathway.Citation67,Citation69 Overexpression of miR-33 in hepatocytes reduced CPT1a and HADHB expression and thereby decreased cellular β-oxidation of fatty acids. Taken together, these results underscore the potential usefulness of anti-miR-33 as a therapeutic target for metabolic disease. Further in vivo studies will be necessary to test this possibility.
miR-378/378* in lipid metabolism
Recently, miR-378/378* was found to play a role in the regulation of lipid metabolism.Citation70 Both are intronic miRNAs cotranscribed within the first intron of the PPARγ coactivator-1 β (PGC-1β) and are highly induced during adipogenesis.Citation70 ST2 mesenchymal precursor cells infected with miR-378/378* had increased lipid droplet size and accumulation of triacylglycerol, accompanied by increased expression of lipogenic genes including Krüppel-like factor 5, fatty acid binding protein 4, fatty acid synthase, stearoyl-coenzymeA desaturase, and resistin. Conversely, knockdown of miR-378/378* in 3T3-L1 adipocytes led to decreased accumulation of triacylglycerol. They also found that miR-378/378* also appeared to increase the transcriptional activity of CCAAT-enhancer-binding protein (C/EBP) α and β on adipocyte gene promoter.
RBPs in lipid metabolism
HuR (human antigen R) is a ubiquitously expressed RBP belonging to Hu/ELAV family and modulates the stability and translational efficiency of mRNAs by interacting with ARE in the 3′-UTR. Gantt and colleagues showed that HuR plays a role during acquisition of the adipocyte phenotype.Citation71 HuR was constitutively expressed and localized predominantly to the nucleus in the preadipocytes, whereas adipogenic stimulus increased the HuR content in the cytosol and rapid formation of HuR complexes containing C/EBPβ mRNA. In the 3T3-L1 cell, suppression of HuR expression resulted in a decrease of C/EBPβ expression and accumulation of lipid droplets and, in turn, an inhibition of adipogenesis, suggesting that HuR functions in establishing the adipocyte phenotype.
Similar to HuR, hematopoietic zinc finger (Hzf) was also found to be required for efficient adipogenesis through the translational regulation of C/EBPα mRNA.Citation72 Hzf was highly expressed in mouse adipose tissue and was markedly induced during adipogenesis. Silencing of Hzf by shRNA in 3T3-L1 cells caused impaired expression of C/EBPα and decreased triglyceride levels and glucose uptake, resulting in attenuated adipogenesis. The effect of Hzf on adipogenesis was mediated by its association with the 3′-UTR of C/EBPα mRNA to enhance its efficient translation and to thereby reinforce adipogenesis. Consistent with studies using cell lines, C/EBPα protein level, but not mRNA level, was lower in Hzf-null mice than in wildtype, suggesting that C/EBPα is translationally regulated by Hzf in vivo. In addition, Hzf-null mice showed an impaired glucose metabolism and reduced insulin sensitivity.
Others
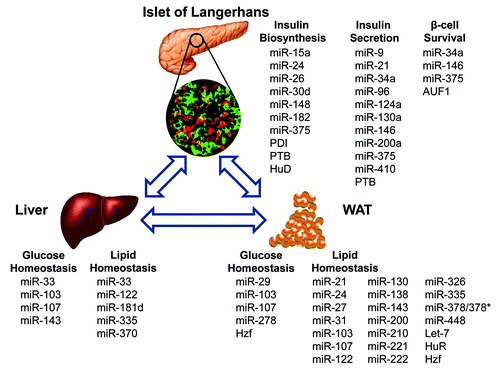
Other miRNAs and RBPs involved in glucose and lipid homeostasis, but not described here, are summarized in .Citation73-Citation99
Table 1. miRNAs and RBPs involved in glucose and lipid homeostasis.
Conclusion
Since metabolic diseases are major public health concerns, the importance of developing methods for managing and treating metabolic diseases is extremely urgent, particularly when one considers the social and economic strain that results if optimal therapy is not available. As the present review shows, numerous post-transcriptional regulators of these metabolic processes have been identified. There is a broad agreement that regulators such as miRNAs and RBPs are linked to the pathogenesis and pathophysiology of metabolic diseases, especially T2DM. Altered patterns of miRNA expression have been seen in T2DM patients,Citation100,Citation101 and a unique miRNA signature was detected in blood of T2DM patientsCitation101 and these molecules are stable in stored samples.Citation100,Citation102 Although the determination of their potential to diagnose T2DM remains to be confirmed by more systematic and large independent studies of well-characterized cohorts of patients, miRNAs circulating in the blood may have the potential to serve as novel biomarkers for the detection and diagnosis of T2DM. Moreover, modifying their behavior by miRNAs or their mimics, as well as inhibitors of miRNA function is also widely recognized and could be the potential therapeutics for targeting metabolic diseases. miRNA activity can be enhanced by transfecting synthetic miRNA mimetics or by using plasmids to transcribe miRNAs or suppressed by antisence oligonucleotides, termed antagomirs,Citation62 whose stability and binding affinity can be increased by chemical modification such as 2′-O-methyl (2′-O-Me), 2′-O-MOE, and LNA.Citation103 Indeed, intravenous administration of antagomirs against miR-16, miR-122, miR-192, and miR-194 resulted in suppression of corresponding miRNA levels in liver, lung, kidney, heart, intestine, fat, skin, bone marrow, muscle, ovaries and adrenals.Citation62 Of relevance to metabolism, several studies have further evaluated the potency of different chemically modified antagomir designs in targeting of miR-122,Citation62-Citation64 miR-103/107,Citation38 and miR-33.Citation65-Citation68 Clinical trials are currently underway that target miR-122 with antisense LNA technology as a therapeutic approach for hypercholesterolemia.Citation104 Besides antagomirs, miRNA spongesCitation105 and small-molecule inhibitorsCitation106 might be an alternative approach to suppress miRNA activity. Despite recent advances in the design of therapeutic strategies for miRNAs, it is important to recognize that miRNAs likely function by simultaneously targeting groups of mRNAs (instead of a single mRNA), and therein lies their effectiveness, as they can jointly affect expression of many proteins within a given pathway. Also, novel and precisely delivery systems of the miRNAs and its inhibitors into the tissue of interest need to be explored and developed. Therefore, a more comprehensive knowledge of the molecular post-transcriptional influence of RBPs and miRNAs in metabolic diseases and better understanding of the upstream factors regulating their expression would be useful so as to design valid therapeutic strategies to treat metabolic diseases. In the future, approaches to target these molecules selectively in a cell- or tissue-specific manner will need to be developed and evaluated carefully for safety and therapeutic effectiveness ().
Acknowledgments
We appreciate M.Gorospe and J.M.Egan for critical reading of the manuscript. W. Kim is supported by the Intramural Research Program of the National Institute on Aging (NIA)/NIH. E.K.Lee is supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (20110013116) and the Catholic Medical Center Research Foundation.
References
- Permutt MA, Wasson J, Cox N. Genetic epidemiology of diabetes. J Clin Invest 2005; 115:1431 - 9; http://dx.doi.org/10.1172/JCI24758; PMID: 15931378
- Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, et al. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med 1993; 329:1988 - 92; http://dx.doi.org/10.1056/NEJM199312303292703; PMID: 8247074
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001; 414:799 - 806; http://dx.doi.org/10.1038/414799a; PMID: 11742412
- Anderson JW, Kendall CW, Jenkins DJ. Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. J Am Coll Nutr 2003; 22:331 - 9; PMID: 14559925
- Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 2005; 433:769 - 73; http://dx.doi.org/10.1038/nature03315; PMID: 15685193
- Chekulaeva M, Filipowicz W. Mechanisms of miRNA-mediated post-transcriptional regulation in animal cells. Curr Opin Cell Biol 2009; 21:452 - 60; http://dx.doi.org/10.1016/j.ceb.2009.04.009; PMID: 19450959
- Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem 2010; 79:351 - 79; http://dx.doi.org/10.1146/annurev-biochem-060308-103103; PMID: 20533884
- Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science 2007; 318:1931 - 4; http://dx.doi.org/10.1126/science.1149460; PMID: 18048652
- Ørom UA, Nielsen FC, Lund AH. MicroRNA-10a binds the 5’UTR of ribosomal protein mRNAs and enhances their translation. Mol Cell 2008; 30:460 - 71; http://dx.doi.org/10.1016/j.molcel.2008.05.001; PMID: 18498749
- Henke JI, Goergen D, Zheng J, Song Y, Schüttler CG, Fehr C, et al. microRNA-122 stimulates translation of hepatitis C virus RNA. EMBO J 2008; 27:3300 - 10; http://dx.doi.org/10.1038/emboj.2008.244; PMID: 19020517
- Abdelmohsen K, Kuwano Y, Kim HH, Gorospe M. Posttranscriptional gene regulation by RNA-binding proteins during oxidative stress: implications for cellular senescence. Biol Chem 2008; 389:243 - 55; http://dx.doi.org/10.1515/BC.2008.022; PMID: 18177264
- Lunde BM, Moore C, Varani G. RNA-binding proteins: modular design for efficient function. Nat Rev Mol Cell Biol 2007; 8:479 - 90; http://dx.doi.org/10.1038/nrm2178; PMID: 17473849
- Lee EK, Gorospe M. Coding region: the neglected post-transcriptional code. RNA Biol 2011; 8:44 - 8; http://dx.doi.org/10.4161/rna.8.1.13863; PMID: 21289484
- Abdelmohsen K, Tominaga K, Lee EK, Srikantan S, Kang MJ, Kim MM, et al. Enhanced translation by Nucleolin via G-rich elements in coding and non-coding regions of target mRNAs. Nucleic Acids Res 2011; 39:8513 - 30; http://dx.doi.org/10.1093/nar/gkr488; PMID: 21737422
- Withers DJ, Gutierrez JS, Towery H, Burks DJ, Ren JM, Previs S, et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature 1998; 391:900 - 4; http://dx.doi.org/10.1038/36116; PMID: 9495343
- Kulkarni RN, Brüning JC, Winnay JN, Postic C, Magnuson MA, Kahn CR. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell 1999; 96:329 - 39; http://dx.doi.org/10.1016/S0092-8674(00)80546-2; PMID: 10025399
- Kulkarni RN, Winnay JN, Daniels M, Brüning JC, Flier SN, Hanahan D, et al. Altered function of insulin receptor substrate-1-deficient mouse islets and cultured beta-cell lines. J Clin Invest 1999; 104:R69 - 75; http://dx.doi.org/10.1172/JCI8339; PMID: 10606633
- Uchida T, Nakamura T, Hashimoto N, Matsuda T, Kotani K, Sakaue H, et al. Deletion of Cdkn1b ameliorates hyperglycemia by maintaining compensatory hyperinsulinemia in diabetic mice. Nat Med 2005; 11:175 - 82; http://dx.doi.org/10.1038/nm1187; PMID: 15685168
- Ebina Y, Ellis L, Jarnagin K, Edery M, Graf L, Clauser E, et al. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell 1985; 40:747 - 58; http://dx.doi.org/10.1016/0092-8674(85)90334-4; PMID: 2859121
- Ullrich A, Bell JR, Chen EY, Herrera R, Petruzzelli LM, Dull TJ, et al. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. Nature 1985; 313:756 - 61; http://dx.doi.org/10.1038/313756a0; PMID: 2983222
- Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, et al. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell 2000; 6:87 - 97; PMID: 10949030
- Fernández AM, Kim JK, Yakar S, Dupont J, Hernandez-Sanchez C, Castle AL, et al. Functional inactivation of the IGF-I and insulin receptors in skeletal muscle causes type 2 diabetes. Genes Dev 2001; 15:1926 - 34; http://dx.doi.org/10.1101/gad.908001; PMID: 11485987
- Miyake K, Ogawa W, Matsumoto M, Nakamura T, Sakaue H, Kasuga M. Hyperinsulinemia, glucose intolerance, and dyslipidemia induced by acute inhibition of phosphoinositide 3-kinase signaling in the liver. J Clin Invest 2002; 110:1483 - 91; PMID: 12438446
- Poy MN, Eliasson L, Krutzfeldt J, Kuwajima S, Ma X, Macdonald PE, et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature 2004; 432:226 - 30; http://dx.doi.org/10.1038/nature03076; PMID: 15538371
- El Ouaamari A, Baroukh N, Martens GA, Lebrun P, Pipeleers D, van Obberghen E. miR-375 targets 3′-phosphoinositide-dependent protein kinase-1 and regulates glucose-induced biological responses in pancreatic beta-cells. Diabetes 2008; 57:2708 - 17; http://dx.doi.org/10.2337/db07-1614; PMID: 18591395
- Keller DM, McWeeney S, Arsenlis A, Drouin J, Wright CV, Wang H, et al. Characterization of pancreatic transcription factor Pdx-1 binding sites using promoter microarray and serial analysis of chromatin occupancy. J Biol Chem 2007; 282:32084 - 92; http://dx.doi.org/10.1074/jbc.M700899200; PMID: 17761679
- Zhao E, Keller MP, Rabaglia ME, Oler AT, Stapleton DS, Schueler KL, et al. Obesity and genetics regulate microRNAs in islets, liver, and adipose of diabetic mice. Mamm Genome 2009; 20:476 - 85; http://dx.doi.org/10.1007/s00335-009-9217-2; PMID: 19727952
- Poy MN, Hausser J, Trajkovski M, Braun M, Collins S, Rorsman P, et al. miR-375 maintains normal pancreatic alpha- and beta-cell mass. Proc Natl Acad Sci U S A 2009; 106:5813 - 8; http://dx.doi.org/10.1073/pnas.0810550106; PMID: 19289822
- Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, et al. RAS is regulated by the let-7 microRNA family. Cell 2005; 120:635 - 47; http://dx.doi.org/10.1016/j.cell.2005.01.014; PMID: 15766527
- Lee YS, Dutta A. The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes Dev 2007; 21:1025 - 30; http://dx.doi.org/10.1101/gad.1540407; PMID: 17437991
- Mayr C, Hemann MT, Bartel DP. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science 2007; 315:1576 - 9; http://dx.doi.org/10.1126/science.1137999; PMID: 17322030
- Kumar MS, Erkeland SJ, Pester RE, Chen CY, Ebert MS, Sharp PA, et al. Suppression of non-small cell lung tumor development by the let-7 microRNA family. Proc Natl Acad Sci U S A 2008; 105:3903 - 8; http://dx.doi.org/10.1073/pnas.0712321105; PMID: 18308936
- Heo I, Joo C, Cho J, Ha M, Han J, Kim VN. Lin28 mediates the terminal uridylation of let-7 precursor MicroRNA. Mol Cell 2008; 32:276 - 84; http://dx.doi.org/10.1016/j.molcel.2008.09.014; PMID: 18951094
- Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science 2008; 320:97 - 100; http://dx.doi.org/10.1126/science.1154040; PMID: 18292307
- Viswanathan SR, Powers JT, Einhorn W, Hoshida Y, Ng TL, Toffanin S, et al. Lin28 promotes transformation and is associated with advanced human malignancies. Nat Genet 2009; 41:843 - 8; http://dx.doi.org/10.1038/ng.392; PMID: 19483683
- Zhu H, Shyh-Chang N, Segrè AV, Shinoda G, Shah SP, Einhorn WS, et al, DIAGRAM Consortium, MAGIC Investigators. The Lin28/let-7 axis regulates glucose metabolism. Cell 2011; 147:81 - 94; http://dx.doi.org/10.1016/j.cell.2011.08.033; PMID: 21962509
- Guay C, Roggli E, Nesca V, Jacovetti C, Regazzi R. Diabetes mellitus, a microRNA-related disease?. Transl Res 2011; 157:253 - 64; http://dx.doi.org/10.1016/j.trsl.2011.01.009; PMID: 21420036
- Trajkovski M, Hausser J, Soutschek J, Bhat B, Akin A, Zavolan M, et al. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature 2011; 474:649 - 53; http://dx.doi.org/10.1038/nature10112; PMID: 21654750
- Jordan SD, Krüger M, Willmes DM, Redemann N, Wunderlich FT, Brönneke HS, et al. Obesity-induced overexpression of miRNA-143 inhibits insulin-stimulated AKT activation and impairs glucose metabolism. Nat Cell Biol 2011; 13:434 - 46; http://dx.doi.org/10.1038/ncb2211; PMID: 21441927
- Esau C, Kang X, Peralta E, Hanson E, Marcusson EG, Ravichandran LV, et al. MicroRNA-143 regulates adipocyte differentiation. J Biol Chem 2004; 279:52361 - 5; http://dx.doi.org/10.1074/jbc.C400438200; PMID: 15504739
- Xie H, Lim B, Lodish HF. MicroRNAs induced during adipogenesis that accelerate fat cell development are downregulated in obesity. Diabetes 2009; 58:1050 - 7; http://dx.doi.org/10.2337/db08-1299; PMID: 19188425
- Takanabe R, Ono K, Abe Y, Takaya T, Horie T, Wada H, et al. Up-regulated expression of microRNA-143 in association with obesity in adipose tissue of mice fed high-fat diet. Biochem Biophys Res Commun 2008; 376:728 - 32; http://dx.doi.org/10.1016/j.bbrc.2008.09.050; PMID: 18809385
- Li S, Chen X, Zhang H, Liang X, Xiang Y, Yu C, et al. Differential expression of microRNAs in mouse liver under aberrant energy metabolic status. J Lipid Res 2009; 50:1756 - 65; http://dx.doi.org/10.1194/jlr.M800509-JLR200; PMID: 19372595
- Gallagher IJ, Scheele C, Keller P, Nielsen AR, Remenyi J, Fischer CP, et al. Integration of microRNA changes in vivo identifies novel molecular features of muscle insulin resistance in type 2 diabetes. Genome Med 2010; 2:9; http://dx.doi.org/10.1186/gm130; PMID: 20353613
- Lee EK, Gorospe M. Minireview: posttranscriptional regulation of the insulin and insulin-like growth factor systems. Endocrinology 2010; 151:1403 - 8; http://dx.doi.org/10.1210/en.2009-1123; PMID: 20032049
- Melkman-Zehavi T, Oren R, Kredo-Russo S, Shapira T, Mandelbaum AD, Rivkin N, et al. miRNAs control insulin content in pancreatic β-cells via downregulation of transcriptional repressors. EMBO J 2011; 30:835 - 45; http://dx.doi.org/10.1038/emboj.2010.361; PMID: 21285947
- Kulkarni SD, Muralidharan B, Panda AC, Bakthavachalu B, Vindu A, Seshadri V. Glucose-stimulated translation regulation of insulin by the 5′ UTR-binding proteins. J Biol Chem 2011; 286:14146 - 56; http://dx.doi.org/10.1074/jbc.M110.190553; PMID: 21357685
- Roggli E, Gattesco S, Pautz A, Regazzi R. Involvement of the RNA-binding protein ARE/poly(U)-binding factor 1 (AUF1) in the cytotoxic effects of proinflammatory cytokines on pancreatic beta cells. [Epup ahead of print] Diabetologia 2011; http://dx.doi.org/10.1007/s00125-011-2399-7; PMID: 22159912
- Tillmar L, Carlsson C, Welsh N. Control of insulin mRNA stability in rat pancreatic islets. Regulatory role of a 3′-untranslated region pyrimidine-rich sequence. J Biol Chem 2002; 277:1099 - 106; http://dx.doi.org/10.1074/jbc.M108340200; PMID: 11696543
- Tillmar L, Welsh N. Hypoxia may increase rat insulin mRNA levels by promoting binding of the polypyrimidine tract-binding protein (PTB) to the pyrimidine-rich insulin mRNA 3′-untranslated region. Mol Med 2002; 8:263 - 72; PMID: 12359957
- Knoch KP, Bergert H, Borgonovo B, Saeger HD, Altkrüger A, Verkade P, et al. Polypyrimidine tract-binding protein promotes insulin secretory granule biogenesis. Nat Cell Biol 2004; 6:207 - 14; http://dx.doi.org/10.1038/ncb1099; PMID: 15039777
- Lee EK, Kim W, Tominaga K, Martindale JL, Yang X, Subaran SS, et al. RNA-binding protein HuD controls insulin translation. Mol Cell 2012; In press http://dx.doi.org/10.1016/j.molcel.2012.01.016; PMID: 22387028
- Szabo A, Dalmau J, Manley G, Rosenfeld M, Wong E, Henson J, et al. HuD, a paraneoplastic encephalomyelitis antigen, contains RNA-binding domains and is homologous to Elav and Sex-lethal. Cell 1991; 67:325 - 33; http://dx.doi.org/10.1016/0092-8674(91)90184-Z; PMID: 1655278
- Okano HJ, Darnell RB. A hierarchy of Hu RNA binding proteins in developing and adult neurons. J Neurosci 1997; 17:3024 - 37; PMID: 9096138
- Grundy SM. Absorption and metabolism of dietary cholesterol. Annu Rev Nutr 1983; 3:71 - 96; http://dx.doi.org/10.1146/annurev.nu.03.070183.000443; PMID: 6357243
- Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 1997; 89:331 - 40; http://dx.doi.org/10.1016/S0092-8674(00)80213-5; PMID: 9150132
- Peet DJ, Janowski BA, Mangelsdorf DJ. The LXRs: a new class of oxysterol receptors. Curr Opin Genet Dev 1998; 8:571 - 5; http://dx.doi.org/10.1016/S0959-437X(98)80013-0; PMID: 9794827
- Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab 2008; 7:365 - 75; http://dx.doi.org/10.1016/j.cmet.2008.03.001; PMID: 18460328
- Schroeder F. Fluorescence probes in metastatic B16 melanoma membranes. Biochim Biophys Acta 1984; 776:299 - 312; http://dx.doi.org/10.1016/0005-2736(84)90219-0; PMID: 6477911
- Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol 1995; 15:551 - 61; http://dx.doi.org/10.1161/01.ATV.15.5.551; PMID: 7749869
- Aikawa M, Libby P. The vulnerable atherosclerotic plaque: pathogenesis and therapeutic approach. Cardiovasc Pathol 2004; 13:125 - 38; http://dx.doi.org/10.1016/S1054-8807(04)00004-3; PMID: 15081469
- Krützfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature 2005; 438:685 - 9; http://dx.doi.org/10.1038/nature04303; PMID: 16258535
- Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab 2006; 3:87 - 98; http://dx.doi.org/10.1016/j.cmet.2006.01.005; PMID: 16459310
- Elmén J, Lindow M, Silahtaroglu A, Bak M, Christensen M, Lind-Thomsen A, et al. Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic Acids Res 2008; 36:1153 - 62; http://dx.doi.org/10.1093/nar/gkm1113; PMID: 18158304
- Marquart TJ, Allen RM, Ory DS, Baldán A. miR-33 links SREBP-2 induction to repression of sterol transporters. Proc Natl Acad Sci U S A 2010; 107:12228 - 32; http://dx.doi.org/10.1073/pnas.1005191107; PMID: 20566875
- Rayner KJ, Suárez Y, Dávalos A, Parathath S, Fitzgerald ML, Tamehiro N, et al. MiR-33 contributes to the regulation of cholesterol homeostasis. Science 2010; 328:1570 - 3; http://dx.doi.org/10.1126/science.1189862; PMID: 20466885
- Dávalos A, Goedeke L, Smibert P, Ramírez CM, Warrier NP, Andreo U, et al. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc Natl Acad Sci U S A 2011; 108:9232 - 7; http://dx.doi.org/10.1073/pnas.1102281108; PMID: 21576456
- Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE, Gerszten RE, et al. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science 2010; 328:1566 - 9; http://dx.doi.org/10.1126/science.1189123; PMID: 20466882
- Gerin I, Clerbaux LA, Haumont O, Lanthier N, Das AK, Burant CF, et al. Expression of miR-33 from an SREBP2 intron inhibits cholesterol export and fatty acid oxidation. J Biol Chem 2010; 285:33652 - 61; http://dx.doi.org/10.1074/jbc.M110.152090; PMID: 20732877
- Gerin I, Bommer GT, McCoin CS, Sousa KM, Krishnan V, MacDougald OA. Roles for miRNA-378/378* in adipocyte gene expression and lipogenesis. Am J Physiol Endocrinol Metab 2010; 299:E198 - 206; PMID: 20484008
- Gantt K, Cherry J, Tenney R, Karschner V, Pekala PH. An early event in adipogenesis, the nuclear selection of the CCAAT enhancer-binding protein beta (C/EBPbeta) mRNA by HuR and its translocation to the cytosol. J Biol Chem 2005; 280:24768 - 74; http://dx.doi.org/10.1074/jbc.M502011200; PMID: 15863502
- Kawagishi H, Wakoh T, Uno H, Maruyama M, Moriya A, Morikawa S, et al. Hzf regulates adipogenesis through translational control of C/EBPalpha. EMBO J 2008; 27:1481 - 90; PMID: 18418387
- Plaisance V, Abderrahmani A, Perret-Menoud V, Jacquemin P, Lemaigre F, Regazzi R. MicroRNA-9 controls the expression of Granuphilin/Slp4 and the secretory response of insulin-producing cells. J Biol Chem 2006; 281:26932 - 42; http://dx.doi.org/10.1074/jbc.M601225200; PMID: 16831872
- Sun LL, Jiang BG, Li WT, Zou JJ, Shi YQ, Liu ZM. MicroRNA-15a positively regulates insulin synthesis by inhibiting uncoupling protein-2 expression. Diabetes Res Clin Pract 2011; 91:94 - 100; http://dx.doi.org/10.1016/j.diabres.2010.11.006; PMID: 21146880
- Roggli E, Britan A, Gattesco S, Lin-Marq N, Abderrahmani A, Meda P, et al. Involvement of microRNAs in the cytotoxic effects exerted by proinflammatory cytokines on pancreatic beta-cells. Diabetes 2010; 59:978 - 86; http://dx.doi.org/10.2337/db09-0881; PMID: 20086228
- Ruan Q, Wang T, Kameswaran V, Wei Q, Johnson DS, Matschinsky F, et al. The microRNA-21-PDCD4 axis prevents type 1 diabetes by blocking pancreatic beta cell death. Proc Natl Acad Sci U S A 2011; 108:12030 - 5; http://dx.doi.org/10.1073/pnas.1101450108; PMID: 21730150
- He A, Zhu L, Gupta N, Chang Y, Fang F. Overexpression of micro ribonucleic acid 29, highly up-regulated in diabetic rats, leads to insulin resistance in 3T3-L1 adipocytes. Mol Endocrinol 2007; 21:2785 - 94; http://dx.doi.org/10.1210/me.2007-0167; PMID: 17652184
- Tang X, Muniappan L, Tang G, Ozcan S. Identification of glucose-regulated miRNAs from pancreatic beta cells reveals a role for miR-30d in insulin transcription. RNA 2009; 15:287 - 93; http://dx.doi.org/10.1261/rna.1211209; PMID: 19096044
- Lovis P, Roggli E, Laybutt DR, Gattesco S, Yang JY, Widmann C, et al. Alterations in microRNA expression contribute to fatty acid-induced pancreatic beta-cell dysfunction. Diabetes 2008; 57:2728 - 36; http://dx.doi.org/10.2337/db07-1252; PMID: 18633110
- Lovis P, Gattesco S, Regazzi R. Regulation of the expression of components of the exocytotic machinery of insulin-secreting cells by microRNAs. Biol Chem 2008; 389:305 - 12; http://dx.doi.org/10.1515/BC.2008.026; PMID: 18177263
- Hennessy E, Clynes M, Jeppesen PB, O’Driscoll L. Identification of microRNAs with a role in glucose stimulated insulin secretion by expression profiling of MIN6 cells. Biochem Biophys Res Commun 2010; 396:457 - 62; http://dx.doi.org/10.1016/j.bbrc.2010.04.116; PMID: 20417623
- Teleman AA, Maitra S, Cohen SM. Drosophila lacking microRNA miR-278 are defective in energy homeostasis. Genes Dev 2006; 20:417 - 22; http://dx.doi.org/10.1101/gad.374406; PMID: 16481470
- Kim YJ, Hwang SJ, Bae YC, Jung JS. MiR-21 regulates adipogenic differentiation through the modulation of TGF-beta signaling in mesenchymal stem cells derived from human adipose tissue. Stem Cells 2009; 27:3093 - 102; PMID: 19816956
- Sun F, Wang J, Pan Q, Yu Y, Zhang Y, Wan Y, et al. Characterization of function and regulation of miR-24-1 and miR-31. Biochem Biophys Res Commun 2009; 380:660 - 5; http://dx.doi.org/10.1016/j.bbrc.2009.01.161; PMID: 19285018
- Lin Q, Gao Z, Alarcon RM, Ye J, Yun Z. A role of miR-27 in the regulation of adipogenesis. FEBS J 2009; 276:2348 - 58; http://dx.doi.org/10.1111/j.1742-4658.2009.06967.x; PMID: 19348006
- Kim SY, Kim AY, Lee HW, Son YH, Lee GY, Lee JW, et al. miR-27a is a negative regulator of adipocyte differentiation via suppressing PPARgamma expression. Biochem Biophys Res Commun 2010; 392:323 - 8; http://dx.doi.org/10.1016/j.bbrc.2010.01.012; PMID: 20060380
- Tang YF, Zhang Y, Li XY, Li C, Tian W, Liu L. Expression of miR-31, miR-125b-5p, and miR-326 in the adipogenic differentiation process of adipose-derived stem cells. OMICS 2009; 13:331 - 6; http://dx.doi.org/10.1089/omi.2009.0017; PMID: 19422302
- Xie H, Lim B, Lodish HF. MicroRNAs induced during adipogenesis that accelerate fat cell development are downregulated in obesity. Diabetes 2009; 58:1050 - 7; http://dx.doi.org/10.2337/db08-1299; PMID: 19188425
- Wilfred BR, Wang WX, Nelson PT. Energizing miRNA research: a review of the role of miRNAs in lipid metabolism, with a prediction that miR-103/107 regulates human metabolic pathways. Mol Genet Metab 2007; 91:209 - 17; http://dx.doi.org/10.1016/j.ymgme.2007.03.011; PMID: 17521938
- Lee EK, Lee MJ, Abdelmohsen K, Kim W, Kim MM, Srikantan S, et al. miR-130 suppresses adipogenesis by inhibiting peroxisome proliferator-activated receptor gamma expression. Mol Cell Biol 2011; 31:626 - 38; http://dx.doi.org/10.1128/MCB.00894-10; PMID: 21135128
- Yang Z, Bian C, Zhou H, Huang S, Wang S, Liao L, et al. MicroRNA hsa-miR-138 inhibits adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells through adenovirus EID-1. Stem Cells Dev 2011; 20:259 - 67; http://dx.doi.org/10.1089/scd.2010.0072; PMID: 20486779
- Esau C, Kang X, Peralta E, Hanson E, Marcusson EG, Ravichandran LV, et al. MicroRNA-143 regulates adipocyte differentiation. J Biol Chem 2004; 279:52361 - 5; http://dx.doi.org/10.1074/jbc.C400438200; PMID: 15504739
- Whittaker R, Loy PA, Sisman E, Suyama E, Aza-Blanc P, Ingermanson RS, et al. Identification of MicroRNAs that control lipid droplet formation and growth in hepatocytes via high-content screening. J Biomol Screen 2010; 15:798 - 805; http://dx.doi.org/10.1177/1087057110374991; PMID: 20639500
- Kennell JA, Gerin I, MacDougald OA, Cadigan KM. The microRNA miR-8 is a conserved negative regulator of Wnt signaling. Proc Natl Acad Sci U S A 2008; 105:15417 - 22; http://dx.doi.org/10.1073/pnas.0807763105; PMID: 18824696
- Qin L, Chen Y, Niu Y, Chen W, Wang Q, Xiao S, et al. A deep investigation into the adipogenesis mechanism: profile of microRNAs regulating adipogenesis by modulating the canonical Wnt/beta-catenin signaling pathway. BMC Genomics 2010; 11:320; http://dx.doi.org/10.1186/1471-2164-11-320; PMID: 20492721
- Nakanishi N, Nakagawa Y, Tokushige N, Aoki N, Matsuzaka T, Ishii K, et al. The up-regulation of microRNA-335 is associated with lipid metabolism in liver and white adipose tissue of genetically obese mice. Biochem Biophys Res Commun 2009; 385:492 - 6; http://dx.doi.org/10.1016/j.bbrc.2009.05.058; PMID: 19460359
- Iliopoulos D, Drosatos K, Hiyama Y, Goldberg IJ, Zannis VI. MicroRNA-370 controls the expression of microRNA-122 and Cpt1alpha and affects lipid metabolism. J Lipid Res 2010; 51:1513 - 23; http://dx.doi.org/10.1194/jlr.M004812; PMID: 20124555
- Kinoshita M, Ono K, Horie T, Nagao K, Nishi H, Kuwabara Y, et al. Regulation of adipocyte differentiation by activation of serotonin (5-HT) receptors 5-HT2AR and 5-HT2CR and involvement of microRNA-448-mediated repression of KLF5. Mol Endocrinol 2010; 24:1978 - 87; http://dx.doi.org/10.1210/me.2010-0054; PMID: 20719859
- Sun T, Fu M, Bookout AL, Kliewer SA, Mangelsdorf DJ. MicroRNA let-7 regulates 3T3-L1 adipogenesis. Mol Endocrinol 2009; 23:925 - 31; http://dx.doi.org/10.1210/me.2008-0298; PMID: 19324969
- Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 2008; 18:997 - 1006; http://dx.doi.org/10.1038/cr.2008.282; PMID: 18766170
- Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res 2010; 107:810 - 7; http://dx.doi.org/10.1161/CIRCRESAHA.110.226357; PMID: 20651284
- Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A 2008; 105:10513 - 8; http://dx.doi.org/10.1073/pnas.0804549105; PMID: 18663219
- Stenvang J, Kauppinen S. MicroRNAs as targets for antisense-based therapeutics. Expert Opin Biol Ther 2008; 8:59 - 81; http://dx.doi.org/10.1517/14712598.8.1.59; PMID: 18081537
- Czech MP, Aouadi M, Tesz GJ. RNAi-based therapeutic strategies for metabolic disease. Nat Rev Endocrinol 2011; 7:473 - 84; http://dx.doi.org/10.1038/nrendo.2011.57; PMID: 21502982
- Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods 2007; 4:721 - 6; http://dx.doi.org/10.1038/nmeth1079; PMID: 17694064
- Gumireddy K, Young DD, Xiong X, Hogenesch JB, Huang Q, Deiters A. Small-molecule inhibitors of microrna miR-21 function. Angew Chem Int Ed Engl 2008; 47:7482 - 4; http://dx.doi.org/10.1002/anie.200801555; PMID: 18712719