Abstract
MicroRNAs are small non-coding RNA regulators of gene expression that play important roles in critical biological processes, including cell division, self-renewal and cell state maintenance. Their deregulation leads to extensive clinical consequences in tumorigenesis. Cancers demonstrate heterogeneity in their cell states implicated in their resistance and resurgence. Apart from proliferating cells, cancers harbor a small proportion of assorted quiescent cells that resist conventional therapeutics and contribute to cancer recurrence. MicroRNA expression, targets, microRNPs (microRNA-protein complexes) and their functions have been demonstrated to be regulated in distinct tumor cell states and as an adaptive response to stress signals in tumor-unfavorable environments. In turn, altered microRNPs and their modified post-transcriptional mechanisms of gene expression may contribute to tumor resistance and influence tumor progression. An understanding of distinct microRNA mechanisms in cancer cells would provide extensive insights into the versatile roles of microRNAs in the perpetuation of tumors and indicate potential therapeutic avenues.
Introduction
MicroRNAs
MicroRNAs are a unique class of small, non-coding, 20- to 24-nt regulatory RNAs that modulate gene expression by recognizing target RNAs via base‐pairing, usually in the untranslated regions (UTR) of mRNAs, and guiding effector microRNPs (microRNA-protein complexes) in a controlled manner.Citation1 Often highly conserved and bioinformatically predicted to target a large range of genes involved in cell division, self-renewal and maintenance of cell states,Citation2-Citation6 their deregulation or aberrant function leads to immense clinical consequences ranging from immune and developmental disorders to cancers.Citation7-Citation11
MicroRNAs and microRNPs are intricately connected with tumorigenesis.Citation10-Citation13 Typically found aberrantly overexpressed or deleted in cancers, microRNAs can function as tumor suppressors or oncogenes to control critical growth regulators and thereby, a network of downstream events to orchestrate multiple effects on cancer progression. Conventional oncogenes and tumor suppressors function as transcriptional regulators of pathways for tumor progression by controlling the expression of microRNAs as in the case of the miR-17–92 cluster, regulated by the oncogene, c-myc, which in turn results in suppression of tumor suppressors such as PTEN and p21CIP.Citation7,Citation13-Citation17 Several microRNAs play critical roles in controlling the cell cycle and thereby influence tumorigenesis. For example, miR-16 was discovered in a chromosomal region deleted in 68% of chronic lymphocytic leukemia; subsequent analyses revealed that the miR-16 family of microRNAs controlled expression of several cell cycle factors and of the anti-apoptotic factor, Bcl2, and thereby, influenced cell cycle arrest and apoptosis.Citation11,Citation12 MicroRNAs have been observed to be stable clinical markers in tissue samples and may enable distinction between cancer subtypes, tissue-specific cancers, and different cancer cell states.Citation18 In addition, circulating microRNAs have been identified in several malignancies and are suggested to influence the tumor microenvironment, surrounding non-cancerous and immune cells and contribute to tumor progression.Citation19-Citation21
Quiescent Cell States in Cancer
Several studies indicate that cells that survive clinical therapy include dormant, quiescent (G0)-like cellsCitation22-Citation25 observed as a small (~1% or less) but clinically relevant population in leukemias and in certain solid tumors with poor survival rates. Quiescence or G0 is a unique, adaptive, non-proliferating state that provides an advantageous escape from harsh situations and chemotherapy that lead to permanent outcomes of tumor-negative environments: senescence, differentiation or apoptosis.Citation26-Citation30 Instead the cell is suspended reversibly in an assortment of transition phases (called G0 state for simplicity) where it can develop and respond adequately to more favorable conditions by returning to the cell cycle.Citation26 Quiescent cells play critical roles in cancer including:
1. Quiescent side populations within tumors. Quiescent cancer cells, including some dormant cancer stem cells in the G0 state, can be isolated as a side population from heterogeneous tumors under conditions such as serum-starvation, hypoxia, specific signaling interference and upon chemotherapeutic ablation of dividing tumor cellsCitation26-Citation28,Citation30-Citation32 and contribute to tumor heterogeneity, resistance and recurrence.
2. Quiescent disseminated and circulating tumor cells. Quiescence is observed among disseminated and circulating tumor cells that evade therapy and the immune system by virtue of their quiescent state to eventually extravasate into tissues and establish metastasisCitation33-Citation35
3. Quiescent tumor associated macrophages (TAMs). Tumor associated macrophages are a heterogeneous assortment of quiescent and activated cells that can produce cytokines and other factors to promote the tumor environment, support establishment of metastasis and induce/maintain quiescent tumor cells, enabling their chemoresistance.Citation36-Citation41
A cell exiting from active proliferation has four non-proliferating options: senescence, apoptosis, differentiation and quiescence, the latter having the advantageous characteristic of reversibility observed in quiescent cancer cells, immune cells, wound healing and germ cells.Citation26-Citation28,Citation42-Citation44 Quiescent states are transient and distinct from G1 and G1 arrest, senescence, differentiation and other states of the cell cycle.Citation26-Citation28,Citation42-Citation45 A block to cell division by several methods in cancer cell lines leads to cell death, G1 arrest or senescence rather than quiescence in cells lacking the capacity to enter the quiescent state, which, in the absence of specific tests and markers to distinguish G0 from G1 arrest and senescence, can misleadingly suggest G0.Citation26,Citation42-Citation44,Citation46-Citation49 Additionally, quiescence is also influenced by cell to cell contact, where replating proliferating cells at high density inhibited entry into quiescence and related microRNA functions.Citation26,Citation43-Citation45 Therefore, true quiescence and associated microRNA functions in G0 cancer cellsCitation46-Citation49 is transiently and less frequently observed. MicroRNAs are required to maintain quiescence in tissues like musclesCitation50 and are involved in maintaining G0 side populations in cancers.Citation2,Citation51-Citation53
Differential characteristics involved in microRNA mechanisms between quiescent and proliferating cells
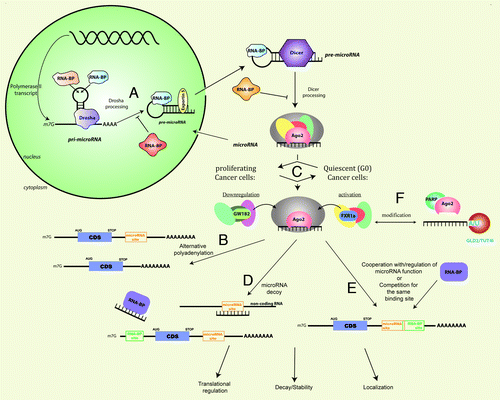
Such reversibly arrested cells are, in contrast, metabolically quite active although distinct from proliferating cells.Citation27,Citation54 G0 cells demonstrate a switch to expression of specific genes that resist tumor-negative conditions, differentiation and cell death to maintain the quiescent state and secure the abilities of the cell to be restored to the proliferative state.Citation27,Citation54 Maintenance of the G0 state can extend from a few hours to several months.Citation26-Citation28,Citation43,Citation44 Quiescent cancer cells have different expression profiles from their proliferating neighbors due to transcriptional differences but also in part due to post-transcriptional microRNA-mediated mechanisms.Citation2,Citation3,Citation51-Citation53,Citation55 In G0, many mRNAs have extended 3′- UTRs, thereby, including microRNA and RNA binding protein regulatory sites.Citation56 Additionally, RNA binding proteins are altered, unmasking previously occupied 3′-UTR microRNA target and other RNA binding regulatory sites.Citation57 The G0-specific included or opened microRNA (and other RNA binding protein sequences like AU-rich elementsCitation46,Citation58) target sites alter gene expression,Citation56 which may play essential roles in tumor progression. Apart from altered mRNA UTRs and UTR-associated RNPs (RNA-protein complexes) that reveal microRNA and other regulatory target sites, G0 states express distinct microRNAs,Citation2,Citation51-Citation53 factors and microRNPs as well as mRNA regulatory mechanisms that are associated with specific gene expression changes,Citation26,Citation27,Citation54 indicating that post-transcriptional regulation plays an important role in maintaining the stateCitation26,Citation42,Citation46-Citation50,Citation54,Citation59 ().
Figure 1. MicroRNA expression and mechanisms are modulated by cancer cell states and stress signals in tumors, leading to misregulation of translation, localization and stability/decay of mRNAs, which influences tumor progression and resistance. MicroRNAs are generally transcribed as pri-microRNA, which are polyadenylated and capped polymerase II transcripts. The pri-microRNAs are processed by the nuclear RNase III enzyme, Drosha, into 70nt pre-microRNAs that are exported to the cytoplasm by Exportin-5. The RNase III enzyme, Dicer, further processes the pre-microRNAs into double-stranded microRNAs that are transferred to Argonaute (AGO). The passenger strand is removed and mature single-stranded microRNAs are incorporated into AGO complexes, forming the microRNP or RISC.Citation66-Citation70 Proliferating and quiescent cancer cells as well as other cancer cell states and distinct types of cancer can alter the composition of associated RNPs for specific microRNAs and mRNAs, which regulate microRNA levels and functions and lead to distinct gene expression outcomes: (a) Processing of microRNAs can be positively or negatively regulated by several RNA binding proteins (RNA-BP) such as KSRP (promotes processing), hnRNP A1 (promotes or represses processing) and Lin28A/Lin28B (inhibitory) by influencing Drosha and Dicer processing.Citation71-Citation81 (b) GW182 interaction with AGO2 elicits repression and downregulationCitation97,Citation103,Citation105,Citation107-Citation109 while GW182 interactions with AGO are altered and reduced in quiescent cellsCitation112-Citation115 leading to loss of repressionCitation124 and activation of specific mRNAs.Citation46-Citation49,Citation119,Citation123,Citation125 (c) The availability of the microRNA target site (microRNA site) on mRNAs can be regulated by alternative polyadenylation, which leads to shortened 3′-UTRs in proliferating cells and therefore, escape from microRNA-mediated control over gene expression; in quiescent cells, longer 3′-UTRs are favored and gene expression is subject to greater regulation by microRNAs and RNA binding proteins.Citation56,Citation62-Citation64,Citation118 (d) RNAs can function as decoys in response to different cancer cell state conditions to activate expression: by microRNAs themselves functioning as RNA decoys to remove repressive RNA-binding proteins from binding and repressing mRNAs via RNA binding sites (RNA-BP site) in distinct cancer cell statesCitation133 or by non-coding RNAs such as pseudogenes that bear similar target sites and sequester the microRNAs from their target mRNAs in different cancer subtypes.Citation135-Citation137 (e) RNA-BPs can regulate microRNA functions both negatively and positively by competing with microRNAs for the same binding sites and by preventing or promoting microRNA functions by regulating target site access.Citation57,Citation89,Citation118,Citation138-Citation141 (f) RNA and protein components of microRNPs can be modified in specific cancer subtypes: through addition of U/A nucleotides at the 3′-ends of microRNAs, which leads to altered microRNA levels and functionsCitation81,Citation143-Citation149; by stress-induced addition of poly(ADP) ribose to AGO2/AGO by PARP, which leads to relief of repression and cleavage and thereby, expression of derepressed target mRNAs.Citation134
Regulated Expression of microRNAs
MicroRNAs are generally transcribed as pri-cursor (pri-microRNA) polyadenylated and capped polymerase II transcripts. The pri-microRNAs are processed by the nuclear RNase III enzyme, Drosha, of the Microprocessor complex into approximately 70nt pre-cursors (pre-microRNA) that are exported by Exportin-5 to the cytoplasm. The RNase III enzyme, Dicer, further processes the pre-microRNAs into double-stranded 22-24mers and mature, single-stranded microRNAs are incorporated into Argonaute (AGO) complexes to form the microRNP or RNA-induced silencing complex (RISC, ).Citation66-Citation70 The conserved terminal loop region of pre-/pri-microRNAs acts as a key regulatory switch for modulating microRNA levels by recruiting distinct RNA binding proteins, dependent on their relative levels in different cancers and cell statesCitation66,Citation70-Citation81 including: heterogeneous nuclear ribonucleoprotein A1 (hnRNP A1) that can promote or abrogate maturation of specific microRNAs,Citation71 the AU-rich element binding mRNA decay factor, KH-type splicing regulatory protein (KSRP) whose misregulation can promote cancer through loss of microRNA expressionCitation72 and Lin28 proteins that inhibit processing at multiple levelsCitation73-Citation81 to maintain the undifferentiated cell state and are overexpressed in several, distinct cancers.Citation81
Distinct microRNA signatures are recognized as potential markers of hypoxia, tissue-specific cancers and cancer subtypes that can be correlated with clinical features, suggesting that microRNA expression is specifically regulated in cancers.Citation10,Citation11 The cell cycle and cell density, which influences the cell cycle state, can directly affect the levels and functions of microRNA processing associated factors such as TRBP and additionally regulate specific microRNA levels by mediating their decay upon cell cycle and cell density changes.Citation82-Citation84 MicroRNA processing related factors such as ARS2, a component of the Cap-binding complex, is essential for proliferation and for primary microRNA processing of several specific microRNAs implicated in transformation in proliferative cells where ARS2 is expressed.Citation85 Apart from direct and specific effects of microRNA levels on distinct tumors, mutations in microRNA processing and regulatory factors such as Dicer (loss of function or deletions), Drosha (a haplotype of 2% frequency associated with increased lung cancer survival), Exportin-5 (a dominant negative mutant present in some cell lines with microsatellite instability) and Lin28 (overexpression leads to decreased let-7, a tumor suppressor) also correlate with tumors of distinct types, either due to effects on the levels of families or groups of microRNAs in these tissues or due to a global loss of microRNA homeostasis, leading to deregulation of both oncogenic and tumor suppressive functions.Citation81,Citation86-Citation88
Altered mRNA 3′-UTRs
In tumors, the 3′-UTR lengths are altered either by chromosomal breaksCitation60,Citation61 and mutations or by alternative polyadenylation,Citation56 thereby disrupting microRNA-mediated control over gene expression, enabling oncogene expression and thus uncontrolled proliferation. A widespread effect of cell state differences between proliferation and quiescence on mRNAs is of alternative polyadenylation leading to preclusion of regulatory sites for RNA binding proteins and microRNAs, which along with a general decreased expression of specific microRNAs in cancers, are predicted to lead to deregulated expression of oncogenes in proliferating cellsCitation56,Citation62-Citation64 (). Increased levels of specific 3′-end processing factors were observed in proliferating cell states compared with their lower levels in non-proliferating and quiescent states, likely enabling greater use of the proximal, weaker, cleavage and polyadenylation sites on mRNAs in proliferating cells and thereby, shortening of the 3′-UTR.Citation56,Citation62-Citation64 Such alternative polyadenylation has been used to distinguish different cancer subtypes.Citation62,Citation63 Mechanisms that alter the inclusion of target sites in mRNAs provide greater opportunity for the diversity in gene expression observed in heterogeneous cancers and may contribute to persistent populations in tumors and tumor subtypes. The ABCG2 transporter gene, critical for chemoresistance, was found to be alternatively polyadenylated forming shorter 3′-UTRs that were overexpressed in a resistant subtype of colon cancer cells; such cells were resistant in comparison to the parent colon cancer cell line, which expressed the longer 3′-UTR isoform and thereby, included target sites and associated microRNA-mediated regulation.Citation65 Variation in expression of such critical, resistance factors and thereby, differential chemoresistance within a tumor population and among tumor subtypes can thus be attributed at least in part, to shortened 3′-UTRs that exclude regulatory target sites and abrogate regulation by microRNAs and other RNA regulatory elements.
Regulated Mechanisms
Apart from altered targets and microRNAs, microRNP factors as well as microRNA-mediated mechanisms are altered in quiescent cells (). Unlike quiescent cells and cells cycling slowly when replated/restarted at lower densities, where several cases of indirect and direct upregulated gene expression have been observed,Citation89,Citation90 high density populations of replated/restarted proliferating cells (cycling cells at high density that are not quiescent) demonstrate increased processing of specific microRNAs and enhanced repression/downregulation.Citation45 These processes suggest that the microRNA population, mechanism and target sites on mRNAs are coordinately manipulated to maintain the cancer cell state: proliferating cells promote oncogene expression and prevent expression of tumor suppressors by precluding microRNA target sites to enable oncogene mRNA expression, blocking the expression of tumor suppressive microRNAs (such as let-7)Citation5 that repress oncogenes as well as by enhancing the levels of oncogenic microRNAs (such as miR-17–92)Citation5 that repress tumor suppressor genes. Importantly, the quiescence and proliferation-induced microRNP characteristics also provide a unique opportunity to target these distinct populations of cancer cells. For example, downregulation of the ABCG2 transporter by overexpression of miR-520h diminishes the presence of tumor side populations along with resistance and invasion typical of micrometastatic cancer cells.Citation55 Therefore, an investigation of distinct, regulated mechanisms of microRNA expression and functions in proliferating and quiescent cancer cell states and in response to stress signaling induced in tumor environments, discussed in this review, would enable a greater understanding of the role of microRNAs in the persistence and progression of cancers.
Conventional and alternative mechanisms of microRNA-mediated regulation of gene expression
MicroRNAs act as targeting molecules via their sequence-specific patterns of base-pairing and guide associated effector RNP complexes to other target RNAs to dictate functional outcomes of target gene expression. The ratio of microRNA to mRNA target levels, which is manipulated by cellular conditions, influences the amplitude of the gene expression effect and range from fine-tuning of gene expression to significant changes. Additionally, the expression and levels of different microRNA families may be regulated by multiple signals, which may produce a combinatorial regulatory effect on many target mRNAs that bear multiple microRNA sites, thus relaying distinct signaling cues and adding greater versatility to gene expression control.Citation1,Citation10,Citation91 The AGO or eukaryotic initiation factor 2C (eIF2C) family of effector proteins associates with microRNAs to form the functional microRNP, which is directed to target RNAs by microRNA base-pairing to the target RNA/mRNA. Complete base-pairing of the microRNA to its target leads to mRNA cleavage, degradation and repression determined by the functional features of the AGO member present in the microRNP.Citation1,Citation10,Citation92,Citation93 Depending on the characteristics of the microRNP-associated effector proteins, partial base-pairing to target sites by the microRNP leads to either the traditional outcome of downregulation/silencing of gene expression by mRNA deadenylationCitation94,Citation95 and translational repressionCitation96,Citation97 or to alternative mechanisms that promote gene expressionCitation89 (). Mechanisms that promote gene expression include direct upregulation of specific messages by microRNAs, microRNA-mediated decoy of translation repressors and indirect gene expression by abrogation of conventional microRNA silencing or relief of repressionCitation48,Citation89,Citation96,Citation97 (, d-f).
Posttranscriptional downregulation by microRNAs
MicroRNAs or siRNAs have predominantly been observed to cause downregulation or silencing by cleavage, repressed translation or deadenylation of the mRNA.Citation92,Citation93,Citation96,Citation97 The key factor, AGO2, bears an RNA-dependent RNase H domain that enables cleavage of completely complementary mRNA sites usually to siRNAsCitation92 but also to some naturally occurring microRNAs such as miR196 that is encoded within the A, B and C clusters of HOX8 genes and downregulates several HOX mRNAs with conserved, extensive complementarity.Citation98 Incomplete complementarity is usually associated with other post-transcriptional mechanisms, predominantly deadenylation and repression.Citation69,Citation96,Citation97
MicroRNAs can mediate repression prior to deadenylation of their messagesCitation94,Citation95,Citation97 and non-polyadenylated transcripts are also repressedCitation95 while deadenylation was demonstrated to be a widespread effect of microRNAs on gene expressionCitation99,Citation100 and ribosome profiling of polyadenylated mRNAs demonstrated deadenylation in the case of several microRNAs.Citation101 Target mRNAs may also be relocalized to storage/decapping bodies called P bodies.Citation96,Citation97 MicroRNAs have been demonstrated to repress translation by several mechanisms including translation initiation, post-initiation/elongation and nascent peptide turnover.Citation96,Citation97,Citation102
GW182
The GW182 family of proteins is conserved from yeast to mammals and is a critical microRNP-associated factor for mediating silencing. GW182 interacts with the AGO family of proteins and with the effector complexes that mediate repression and deadenylation in mammalian cells. AGO2 recruitment of GW182 was demonstrated to be essential for downregulation by deadenylation and translation repression in mammalian cells.Citation103-Citation107 Tethering GW182 is sufficient for repression and deadenylation suggesting that GW182 is a primary effector of repression recruited by AGO/microRNAs to specific targets.Citation103,Citation108,Citation109 GW182 can interact with poly(A) binding protein (PABP) and can recruit the CCR4-CAF1 and PAN2-PAN3 deadenylase complexes directly, as well as the PABP regulatory E3 ubiquitin ligase, EDDCitation110 and mediates deadenylationCitation106,Citation107 and repression.Citation109,Citation111 GW182 was demonstrated to mediate both deadenylation/PABP -dependent and -independent repression.Citation109-Citation111
Regulation by the cell cycle and density in proliferating cells
Core microRNP silencing factors as well as associated factors appear regulated by cell cycle states. GW bodies appear regulated across the cell cycleCitation112-Citation114 and are reduced in quiescence, predicting an altered role in quiescent and quiescent-like cells.Citation46,Citation49,Citation115 GW bodies are not required for downregulation but are considered a consequence of the downregulation functions of microRNPs.Citation116 GW bodies marked by GW182 but not P bodies marked by decapping enzymes were found to decline in G1, increase in S and G2 phases and appear reduced and diffused in G0 cells.Citation46,Citation112-Citation114 Repression is observed to be enhanced with synchronized replated cells in the S/G2 phases of the cell cycleCitation47 and with replated/restarted high density cells that are actively growing within the cell cycle (but not quiescent or low density cells that are cycling slowly in the absence of cell to cell contact)Citation45,Citation47 indicating regulation by the cell cycle and cell density.
The RNA binding Pumilio 1 (Pum1)Citation117 promotes microRNA repressive functions, in part by altering target mRNA structural conformation, thereby enabling access to repressive mechanisms like microRNAs. Pum1 binds specific target sequences on mRNAs, such as p27KIP1, in a cell cycle regulated manner. The Pum1-induced conformational alteration of the mRNA promotes microRNA access and consequent repression of p27 mRNA, exclusively in cycling cells but not in arrested cells.Citation57 Another target of Pumilio is the oncogene, E2F3, an important transcription regulator that functions as a powerful driver of proliferation and is overexpressed in many cancers. Pumilio represses E2F3 directly as well as enhances the activity of many microRNAs targeting E2F3. In cancers like bladder cancer and multiple tumor cell lines, such microRNAs are selectively downregulated or the 3′-UTR of E2F3 is shortened by alternative polyadenylation to remove the Pumilio sites and thus prevent Pumilio and Pumilio-enhanced microRNA -mediated repression.Citation118 Pum1 is phosphorylated in cycling cells, enabling its ability to bind RNA while the RNA binding and overall stability of Pum1 is reduced in arrested cells. The inability of Pum1 to bind in arrested cells permits the RNA stem loop structure of p27 mRNA 3′-UTR to block microRNA access and thereby cause relief of repression.Citation57 Therefore, by activating the RNA binding abilities of repression-promoting regulators like Pum1 specifically in the proliferative state, specific tumor suppressors, like p27, can be suppressed by microRNA-mediated repression, perpetuating the proliferative state. Concurrently, by precluding the mRNA binding sites for Pum1, oncogene mRNAs, like E2F3 mRNA, are relieved of Pumilio-enhanced microRNA-mediated repression and are expressed, leading to a coordinated promotion of proliferation.
Direct posttranscriptional upregulation
Activation has been observed in a growing array of studies with specific mRNAs and microRNAs in distinct cellular conditions like G0, suggesting that activation by microRNAs is a regulated pathway of gene expression by microRNAs. Specific genes expressed in G0, are upregulated while others, particularly associated with the cell cycle, are downregulated.Citation48-Citation50,Citation89,Citation119,Citation120 The alteration in microRNA profile and specific gene expression observed in G0 possibly creates a unique advantage for cancer cells to maintain their state and escape therapeutic targeting of dividing tumor cells.
A modified microRNP comprising AGO2 and a specific isoform of Fragile-X-Mental Retardation Related protein 1 (FXR1), called FXR1-iso-a, along with certain microRNAs in G0 like miR369–3p, associates with and mediates translation activation of specific mRNAs, such as TNFα, in a few quiescent cell lines.Citation46-Citation49 FXR1 isoforms can bind the TNFα ARE and the FXR1 knockout mouse demonstrated cytokine deregulation, muscle wasting and neonatal death, suggestive of post-transcriptional deregulation of multiple cytokine targets including TNFα by FXR1 isoforms.Citation121 Importantly, overexpression of FXR1-iso-a, leads to translation activation in low density proliferating cells, suggesting that modification of the microRNP via association of AGO2 with FXR1-iso-a leads to mRNA recruitment to polysomes in G0.Citation46 The upregulation of certain cytokines, signaling and growth regulatory factors, obtained by inducing G0 in cell lines is relevant to cytokine expression in quiescent monocytesCitation59 and dendritic cellsCitation90 as well as in tumor associated macrophages. Monocytes extravasating into tissues, prior to differentiation into macrophages, undergo a G0-like arrest where TNFα and other cytokines are increased post-transcriptionally via their AREs prior to differentiation into macrophages; these effects can be reproduced by inducing G0 in monocytes in vitro in cell cultures.Citation59 Tumor associated macrophages produce cytokines and factors like TNFα that assist circulating some tumor cells, promote metastasis and induce quiescence in tumor cells, enabling their chemoresistance.Citation36-Citation41
The mTOR pathway responds to nutrient and growth signals to regulate catabolic and anabolic processes, in particular, by regulating general and specific mRNA translation. Signaling control over mTOR is often deregulated in cancers, resulting in hyperactive mTOR signaling. The mTOR pathway regulates translation of 5′-terminal oligopyrimidine tract (TOP) mRNAs that usually encode ribosomal protein and other protein synthesis related factor mRNAs bearing the TOP sequence; translation is repressed upon cell cycle arrest at various points and upon nutritional deprivation.Citation122 MiR-10a binds such target ribosomal protein mRNAs immediately downstream of the TOP sequence at non-canonical target sites, alleviates TOP-mediated repression and stimulates translation in response to treatments that activate TOP mRNA translation, such as anisomycin stress inducement or overexpression of a mutant activated RAS observed in cancers. The stimulated translation is inhibited by rapamycin (inhibitor of mTOR), suggesting involvement of the mTOR pathway.Citation123
In quiescent, confluent RK3E cells, miR206 levels increased and caused upregulated translation of KLF4 mRNA while in cycling cells, miR344 levels increased and caused repression of KLF4 mRNA and 3′UTR reportersCitation119 at overlapping microRNA target sites. MiR-206 can repress KLF4 mRNA expression but only in proliferating breast cancer cells and not in normal immortalized MCF10A cells or confluent RK3E cells, suggesting that inducing miR-206 in quiescent cells leads to specific translation upregulation of KLF4 in response to the non-proliferative, quiescent state. Since KLF4 induces its positive regulator miR-206 as does G0, the positive feedback regulation may enable distinct effects of KLF4 in quiescent compared to proliferating cells: prolonging the doubling time in mesenchymal cells, promoting epithelial differentiation in normal cells and a malignant, mesenchymal-like loss of epithelial phenotype in tumor cells. These studies demonstrate that under quiescent conditions, specific G0-modified mRNAs with G0-induced microRNAs/microRNPs are capable of functioning as translation upregulatory complexes.
Quiescence-induced alterations in microRNPs and translation mechanism
In quiescent cells, the essential AGO2 associated repressor, GW182,Citation97,Citation103,Citation105,Citation107 was demonstrated to be altered in its interaction with AGOCitation112-Citation115 and is marked by a decrease in GW bodiesCitation112-Citation114 although the bodies are not essential for repression.Citation116 Abrogating GW182 or its interaction with AGO2 leads to a loss or relief of repressionCitation124 or to activation as in the case of dAGO2 (a Drosophila AGO ortholog that does not interact with GW182) specifically with unadenylated reporters,Citation125 suggesting that the interaction of AGO2 with GW182 is altered on select transcripts in G0 to mediate translation activation.Citation46,Citation49
A second characteristic, at least on some target mRNAs, is the recruitment of an altered translation activating microRNP complex, AGO2-FXR1-iso-a, which leads to relocalization of the mRNA to polysomesCitation46,Citation48 ( activation). FXR1 has been demonstrated to be associated with ribosomesCitation126 and FXR1-iso-a in particular, on overexpression enhances translation.Citation46
Third, in several examples of translation activation, the activation complex fails to interact with GW182Citation46,Citation115,Citation125 and the target mRNAs lack a poly(A) tailCitation125 or have short poly(A) tails due to general poly(A) shortening,Citation127 thereby, potentially altering PABP-mediated interactions in such situations. Deadenylation activity by Poly(A) Ribonuclease (PARN) is enhanced in quiescent mammalian cells,Citation127 suggesting a distinctive mechanism of translation activation by microRNAs of poly(A) tail shortened mRNAs, as indicated by some of the studies on microRNA-mediated translation upregulation.Citation89,Citation125
Fourthly, upregulation has been observed with transcripts and cellular conditions that involve specialized alternative translation mechanism features: IRES and a lack of a cap and poly(A) tail with dAGO2 in Drosophila extracts and with HCV, a 5′-TOP sequence and the mTOR pathway, short poly (A) mRNAs and conditions, such as oocytes and G0 cells, where general translation is reconfigured to enable specific translation instead.Citation89 AGO and AGO-related PIWI proteins like MILI have been demonstrated to associate with general translation initiation factors,Citation128,Citation129 directly or via other RNA binding proteins, enabling recruitment of the ribosomeCitation130,Citation131 and consequent translation activation. These features suggest that activation involves alternative translation mechanisms for specific gene expression in distinct conditions.
Finally, G0 conditions also display downregulation of expressionCitation50,Citation89,Citation120 especially of cell cycle factors and other such growth arrest-refractory genesCitation26,Citation54 that must be depleted in addition to expression of G0 maintenance factors to perpetuate the quiescence state. Activation in G0 occurs with specific G0-expressed microRNAs and G0-expressed or -mobilized mRNAs that would be biologically required for G0-related functions. Activation was also observed by expressing tethered AGO2 or FXR1-iso-a or select, synthetic microRNAs with their corresponding reporters in G0 cells.Citation46,Citation48 Since free, unbound AGO2 is limiting in the mammalian cell,Citation132 these data suggest that specific G0-induced or mobilized mRNAs/ microRNAs would have the opportunity to be bound by new AGO complexes associated with different cofactors due to altered GW182 interaction in G0, alleviating repressive effects in G0 and mediating upregulation instead.Citation46,Citation48,Citation112,Citation114 These examples indicate that specific mRNAs and microRNAs induced or mobilized in specific G0 cellular conditions, such as miR-369–3pCitation46,Citation48 and miR-206Citation119 expressed in serum-starved or confluent G0 cells, may be recruited into distinct microRNPs that promote activation of specific G0-expressed mRNAs, required for the G0 state, while repression ensues with G0-induced microRNAs on other pre-existing mRNAs such as those encoding proliferation related genes that need to be suppressed to maintain the G0 state.Citation50,Citation89,Citation120
MicroRNA-mediated decoy of repressive proteins
A new functional role for microRNAs was recently demonstrated as decoy RNAs that remove repressive proteins, preventing them from accessing their target mRNAs, which are then expressed (, microRNA binding RNA-BP). MiR-328 expression rescues differentiation and impairs survival of leukemic blasts in blast crisis chronic myelogenous leukemia (CML-BC), where the microRNA levels are usually decreased. MiR-328 exerts dual mechanisms to lead to a common biological consequence of promoting differentiation and inhibiting tumor progression: the microRNA binds and removes a repressive protein, hnRNP E2, from its target mRNA, C/EBPα, whose expression is then upregulated in a seed sequence- and microRNA-target binding independent manner,Citation133 promoting differentiation; however, miR-328 also functions via the normal seed sequence based target mRNA binding, repressing the mRNA encoding the survival factor for leukemic blasts, PIM1, to promote differentiation. Thus, miR-328 employs a novel mechanism in addition to its normal microRNA function and contributes to differentiation, via both positive and negative regulation of gene expression.Citation133 Therefore, microRNAs control pathways and biological outcomes in cancer not only by targeting multiple target genes but also through distinct mechanisms operating on specific transcripts and factors to lead to a common biological purpose. Whether other microRNAs also exert such concurrent mechanisms to elicit a shared outcome in addition to their defined sequence-specific, base-pairing mediated roles remains to be investigated.
Indirect gene expression by relief of repression
Apart from direct upregulation of select transcripts by specific microRNAs in G0 and other distinct conditions, indirect relief of repression is an alternate mechanism that also mediates gene expression where downregulation by microRNAs is abrogated. Relief of repression is regulated by cell cycle state and stress conditions associated with cancers.Citation96,Citation97,Citation134 Many RNAs and RNA-binding proteins are expressed specifically in cancer cell states, subtypes and conditions which, along with microRNP modifications induced by cell cycle state and stress conditions, alter microRNA/microRNP expression and functions and alleviate microRNA-mediated repression thereby, manipulating cancer progression.
Pseudogene transcripts and non-coding RNAs that bear target site sequences of specific oncogene and tumor suppressor mRNAs, function as microRNA regulators by competing with these mRNAs for microRNA binding and sequestration, resulting in alleviation of repression (, microRNAs sequestered by non-coding RNA) and increased expression of critical oncogenes or tumor suppressors.Citation135-Citation137 RNA-binding proteins like HuR either alter mRNA conformation or overall mRNP formation to control access and thereby, recognition of the target sites by microRNAs ().Citation57,Citation138,Citation139 These functions of RNA binding proteins are regulated, microRNA/mRNA specific and can also promote microRNA functionsCitation140 in a regulated manner in response to cancer cell state and stress conditions.Citation138 Alternatively, microRNAs compete directly with RNA binding proteins for the target sites that overlap with RNA binding protein target sequences or structures leading to two outcomes: stabilization and increased expression by increased mRNA levels due to microRNAs preventing access to decay factors or downregulation of expression by microRNAs along with their preclusion of stabilizing factors.Citation89,Citation141
Stress response in tumors, in particular in response to harsh chemotherapy, can trigger either apoptosis or survival depending on the modifications on the microRNP. In response to stress, AGO and target mRNAs are relocalized from P bodiesCitation139 to a lesser extent to stress granulesCitation142 and primarily to the cytoplasm where the mRNAs are expressed due to suppression of microRNA-mediated repression and cleavage of target mRNAs by poly-ADP ribose polymerase mediated modifications of AGO,Citation134 an important, therapeutic target in breast, ovarian, uterine and hematopoietic cancers (). 3′-end modification (adenylation or uridylation) is extensively prevalent among microRNAs of several species.Citation143-Citation149 Interestingly, while in cancer cell lines, uridylation has been associated with degradation intermediates, in non-proliferating tissues, uridylation is observed yet the microRNA levels are abundant,Citation146 suggesting that uridylation may have other undiscovered functions (), that are regulated by non-proliferative conditions. A recent study demonstrated that depletion of the uridylase, TUT4/Zcchc11, had tumor-inhibitory effects in human HER2 positive breast tumors but not in triple negative breast tumors, correlating with the expression of specific microRNA processing regulator proteins.Citation81 These results highlight the importance of understanding microRNA-regulatory mechanisms observed in distinct cancer states and subtypes for developing precise clinical targets and improved therapeutic options.
Conclusions
MicroRNAs demonstrate distinct mechanisms of post-transcriptional regulation of their levels and functions in control over target gene expression in different cancer cell states within tumor populations and in response to the signals in the tumor environment. These regulated, microRNA mechanisms are produced as a result of specific manipulations by RNAs and RNA-binding proteins or by modifications to the mRNA or microRNP; the regulators and modulatory events are themselves controlled by specific cancer state conditions. These mechanistic dynamics provide opportunities for greater specificity and versatile gene expression that enable tumor persistence and progression. An in-depth understanding of microRNA-mediated mechanisms and their regulation in distinct cancer cell states and in response to the tumor environment will provide highly specific markers, clinical targets and advance therapeutic options.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors and this review were supported by a Cancer Research Investigator Award, the D. and M-E Ryder Award and a Smith Family Foundation Award to S.V.
Reference List
- Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev 2006; 20:515 - 24; http://dx.doi.org/10.1101/gad.1399806; PMID: 16510870
- Ivey KN, Srivastava D. MicroRNAs as regulators of differentiation and cell fate decisions. Cell Stem Cell 2010; 7:36 - 41; http://dx.doi.org/10.1016/j.stem.2010.06.012; PMID: 20621048
- Munker R, Calin GA. MicroRNA profiling in cancer. Clin Sci (Lond) 2011; 121:141 - 58; http://dx.doi.org/10.1042/CS20110005; PMID: 21526983
- Subramanyam D, Blelloch R. From microRNAs to targets: pathway discovery in cell fate transitions. Curr Opin Genet Dev 2011; 21:498 - 503; http://dx.doi.org/10.1016/j.gde.2011.04.011; PMID: 21636265
- Kasinski AL, Slack FJ. Epigenetics and genetics. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nat Rev Cancer 2011; 11:849 - 64; http://dx.doi.org/10.1038/nrc3166; PMID: 22113163
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009; 136:215 - 33; http://dx.doi.org/10.1016/j.cell.2009.01.002; PMID: 19167326
- Hwang HW, Mendell JT. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br J Cancer 2006; 94:776 - 80; http://dx.doi.org/10.1038/sj.bjc.6603023; PMID: 16495913
- Taft RJ, Pang KC, Mercer TR, Dinger M, Mattick JS. Non-coding RNAs: regulators of disease. J Pathol 2010; 220:126 - 39; http://dx.doi.org/10.1002/path.2638; PMID: 19882673
- Le Quesne JP, Spriggs KA, Bushell M, Willis AE. Dysregulation of protein synthesis and disease. J Pathol 2010; 220:140 - 51; PMID: 19827082
- Farazi TA, Spitzer JI, Morozov P, Tuschl T. miRNAs in human cancer. J Pathol 2011; 223:102 - 15; http://dx.doi.org/10.1002/path.2806; PMID: 21125669
- Nana-Sinkam SP, Croce CM. MicroRNAs as therapeutic targets in cancer. Transl Res 2011; 157:216 - 25; http://dx.doi.org/10.1016/j.trsl.2011.01.013; PMID: 21420032
- Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A 2002; 99:15524 - 9; http://dx.doi.org/10.1073/pnas.242606799; PMID: 12434020
- Conkrite K, Sundby M, Mukai S, Thomson JM, Mu D, Hammond SM, et al. miR-17~92 cooperates with RB pathway mutations to promote retinoblastoma. Genes Dev 2011; 25:1734 - 45; http://dx.doi.org/10.1101/gad.17027411; PMID: 21816922
- O’Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature 2005; 435:839 - 43; http://dx.doi.org/10.1038/nature03677; PMID: 15944709
- Olive V, Bennett MJ, Walker JC, Ma C, Jiang I, Cordon-Cardo C, et al. miR-19 is a key oncogenic component of mir-17-92. Genes Dev 2009; 23:2839 - 49; http://dx.doi.org/10.1101/gad.1861409; PMID: 20008935
- Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J, et al. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol 2008; 9:405 - 14; http://dx.doi.org/10.1038/ni1575; PMID: 18327259
- Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell 2008; 132:875 - 86; http://dx.doi.org/10.1016/j.cell.2008.02.019; PMID: 18329372
- Zoon CK, Starker EQ, Wilson AM, Emmert-Buck MR, Libutti SK, Tangrea MA. Current molecular diagnostics of breast cancer and the potential incorporation of microRNA. Expert Rev Mol Diagn 2009; 9:455 - 67; http://dx.doi.org/10.1586/erm.09.25; PMID: 19580430
- Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol 2009; 11:1143 - 9; http://dx.doi.org/10.1038/ncb1929; PMID: 19684575
- Lee YS, Pressman S, Andress AP, Kim K, White JL, Cassidy JJ, et al. Silencing by small RNAs is linked to endosomal trafficking. Nat Cell Biol 2009; 11:1150 - 6; http://dx.doi.org/10.1038/ncb1930; PMID: 19684574
- Cortez MA, Bueso-Ramos C, Ferdin J, Lopez-Berestein G, Sood AK, Calin GA. MicroRNAs in body fluids--the mix of hormones and biomarkers. Nat Rev Clin Oncol 2011; 8:467 - 77; http://dx.doi.org/10.1038/nrclinonc.2011.76; PMID: 21647195
- Li L, Bhatia R. Stem cell quiescence. Clin Cancer Res 2011; 17:4936 - 41; http://dx.doi.org/10.1158/1078-0432.CCR-10-1499; PMID: 21593194
- Lindeman GJ, Visvader JE. Insights into the cell of origin in breast cancer and breast cancer stem cells. Asia Pac J Clin Oncol 2010; 6:89 - 97; http://dx.doi.org/10.1111/j.1743-7563.2010.01279.x; PMID: 20565420
- Nicholson E, Holyoake T. The chronic myeloid leukemia stem cell. Clin Lymphoma Myeloma 2009; 9:Suppl 4 S376 - 81; http://dx.doi.org/10.3816/CLM.2009.s.037; PMID: 20007106
- Besançon R, Valsesia-Wittmann S, Puisieux A, Caron de Fromentel C, Maguer-Satta V. Cancer stem cells: the emerging challenge of drug targeting. Curr Med Chem 2009; 16:394 - 416; http://dx.doi.org/10.2174/092986709787315531; PMID: 19199913
- Coller HA, Sang L, Roberts JM. A new description of cellular quiescence. PLoS Biol 2006; 4:e83; http://dx.doi.org/10.1371/journal.pbio.0040083; PMID: 16509772
- Sang L, Coller HA, Roberts JM. Control of the reversibility of cellular quiescence by the transcriptional repressor HES1. Science 2008; 321:1095 - 100; http://dx.doi.org/10.1126/science.1155998; PMID: 18719287
- Tavaluc RT, Hart LS, Dicker DT, El-Deiry WS. Effects of low confluency, serum starvation and hypoxia on the side population of cancer cell lines. Cell Cycle 2007; 6:2554 - 62; http://dx.doi.org/10.4161/cc.6.20.4911; PMID: 17912032
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646 - 74; http://dx.doi.org/10.1016/j.cell.2011.02.013; PMID: 21376230
- Zheng X, Seshire A, Rüster B, Bug G, Beissert T, Puccetti E, et al. Arsenic but not all-trans retinoic acid overcomes the aberrant stem cell capacity of PML/RARalpha-positive leukemic stem cells. Haematologica 2007; 92:323 - 31; http://dx.doi.org/10.3324/haematol.10541; PMID: 17339181
- Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 2009; 138:645 - 59; http://dx.doi.org/10.1016/j.cell.2009.06.034; PMID: 19682730
- Dey-Guha I, Wolfer A, Yeh AC, G Albeck J, Darp R, Leon E, et al. Asymmetric cancer cell division regulated by AKT. Proc Natl Acad Sci U S A 2011; 108:12845 - 50; http://dx.doi.org/10.1073/pnas.1109632108; PMID: 21757645
- Pantel K, Alix-Panabières C, Riethdorf S. Cancer micrometastases. Nat Rev Clin Oncol 2009; 6:339 - 51; http://dx.doi.org/10.1038/nrclinonc.2009.44; PMID: 19399023
- Riethdorf S, Wikman H, Pantel K. Review: Biological relevance of disseminated tumor cells in cancer patients. Int J Cancer 2008; 123:1991 - 2006; http://dx.doi.org/10.1002/ijc.23825; PMID: 18712708
- Yu M, Stott S, Toner M, Maheswaran S, Haber DA. Circulating tumor cells: approaches to isolation and characterization. J Cell Biol 2011; 192:373 - 82; http://dx.doi.org/10.1083/jcb.201010021; PMID: 21300848
- Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, et al. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One 2009; 4:e6562; http://dx.doi.org/10.1371/journal.pone.0006562; PMID: 19668347
- Prewitt TW, Matthews W, Chaudhri G, Pogrebniak HW, Pass HI. Tumor necrosis factor induces doxorubicin resistance to lung cancer cells in vitro. J Thorac Cardiovasc Surg 1994; 107:43 - 9; PMID: 8283917
- Jinushi M, Chiba S, Yoshiyama H, Masutomi K, Kinoshita I, Dosaka-Akita H, et al. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc Natl Acad Sci U S A 2011; 108:12425 - 30; http://dx.doi.org/10.1073/pnas.1106645108; PMID: 21746895
- Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol 2012; 33:119 - 26; http://dx.doi.org/10.1016/j.it.2011.12.001; PMID: 22277903
- Dirkx AE, Oude Egbrink MG, Wagstaff J, Griffioen AW. Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. J Leukoc Biol 2006; 80:1183 - 96; http://dx.doi.org/10.1189/jlb.0905495; PMID: 16997855
- Laoui D, Movahedi K, Van Overmeire E, Van den Bossche J, Schouppe E, Mommer C, et al. Tumor-associated macrophages in breast cancer: distinct subsets, distinct functions. Int J Dev Biol 2011; 55:861 - 7; http://dx.doi.org/10.1387/ijdb.113371dl; PMID: 22161841
- Blomen VA, Boonstra J. Cell fate determination during G1 phase progression. Cell Mol Life Sci 2007; 64:3084 - 104; http://dx.doi.org/10.1007/s00018-007-7271-z; PMID: 17891333
- Friedman DL. Role of cyclic nucleotides in cell growth and differentiation. Physiol Rev 1976; 56:652 - 708; PMID: 185633
- Schorl C, Sedivy JM. Analysis of cell cycle phases and progression in cultured mammalian cells. Methods 2007; 41:143 - 50; http://dx.doi.org/10.1016/j.ymeth.2006.07.022; PMID: 17189856
- Hwang HW, Wentzel EA, Mendell JT. Cell-cell contact globally activates microRNA biogenesis. Proc Natl Acad Sci U S A 2009; 106:7016 - 21; http://dx.doi.org/10.1073/pnas.0811523106; PMID: 19359480
- Vasudevan S, Steitz JA. AU-rich-element-mediated upregulation of translation by FXR1 and Argonaute 2. Cell 2007; 128:1105 - 18; http://dx.doi.org/10.1016/j.cell.2007.01.038; PMID: 17382880
- Vasudevan S, Tong Y, Steitz JA. Cell-cycle control of microRNA-mediated translation regulation. Cell Cycle 2008; 7:1545 - 9; http://dx.doi.org/10.4161/cc.7.11.6018; PMID: 18469529
- Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science 2007; 318:1931 - 4; http://dx.doi.org/10.1126/science.1149460; PMID: 18048652
- Mortensen RD, Serra M, Steitz JA, Vasudevan S. Posttranscriptional activation of gene expression in Xenopus laevis oocytes by microRNA-protein complexes (microRNPs). Proc Natl Acad Sci U S A 2011; 108:8281 - 6; http://dx.doi.org/10.1073/pnas.1105401108; PMID: 21536868
- Cheung TH, Quach NL, Charville GW, Liu L, Park L, Edalati A, et al. Maintenance of muscle stem-cell quiescence by microRNA-489. Nature 2012; 482:524 - 8; http://dx.doi.org/10.1038/nature10834; PMID: 22358842
- Li R, Qian N, Tao K, You N, Wang X, Dou K. MicroRNAs involved in neoplastic transformation of liver cancer stem cells. J Exp Clin Cancer Res 2010; 29:169; http://dx.doi.org/10.1186/1756-9966-29-169; PMID: 21176238
- Nagata Y, Maesawa C, Tada H, Takikawa Y, Yashima-Abo A, Masuda T. Differential microRNA expression between bone marrow side population cells and hepatocytes in adult mice. Int J Mol Med 2009; 24:35 - 43; PMID: 19513532
- Misawa A, Katayama R, Koike S, Tomida A, Watanabe T, Fujita N. AP-1-Dependent miR-21 expression contributes to chemoresistance in cancer stem cell-like SP cells. Oncol Res 2010; 19:23 - 33; http://dx.doi.org/10.3727/096504010X12828372551759; PMID: 21141738
- Lemons JM, Feng XJ, Bennett BD, Legesse-Miller A, Johnson EL, Raitman I, et al. Quiescent fibroblasts exhibit high metabolic activity. PLoS Biol 2010; 8:e1000514; http://dx.doi.org/10.1371/journal.pbio.1000514; PMID: 21049082
- Wang F, Xue X, Wei J, An Y, Yao J, Cai H, et al. hsa-miR-520h downregulates ABCG2 in pancreatic cancer cells to inhibit migration, invasion, and side populations. Br J Cancer 2010; 103:567 - 74; http://dx.doi.org/10.1038/sj.bjc.6605724; PMID: 20628378
- Sandberg R, Neilson JR, Sarma A, Sharp PA, Burge CB. Proliferating cells express mRNAs with shortened 3′ untranslated regions and fewer microRNA target sites. Science 2008; 320:1643 - 7; http://dx.doi.org/10.1126/science.1155390; PMID: 18566288
- Kedde M, van Kouwenhove M, Zwart W, Oude Vrielink JA, Elkon R, Agami R. A Pumilio-induced RNA structure switch in p27-3′ UTR controls miR-221 and miR-222 accessibility. Nat Cell Biol 2010; 12:1014 - 20; http://dx.doi.org/10.1038/ncb2105; PMID: 20818387
- Espel E. The role of the AU-rich elements of mRNAs in controlling translation. Semin Cell Dev Biol 2005; 16:59 - 67; http://dx.doi.org/10.1016/j.semcdb.2004.11.008; PMID: 15659340
- Sirenko O, Böcker U, Morris JS, Haskill JS, Watson JM. IL-1 beta transcript stability in monocytes is linked to cytoskeletal reorganization and the availability of mRNA degradation factors. Immunol Cell Biol 2002; 80:328 - 39; http://dx.doi.org/10.1046/j.1440-1711.2002.01085.x; PMID: 12121221
- Lee YS, Dutta A. The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes Dev 2007; 21:1025 - 30; http://dx.doi.org/10.1101/gad.1540407; PMID: 17437991
- Mayr C, Hemann MT, Bartel DP. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science 2007; 315:1576 - 9; http://dx.doi.org/10.1126/science.1137999; PMID: 17322030
- Mayr C, Bartel DP. Widespread shortening of 3’UTRs by alternative cleavage and polyadenylation activates oncogenes in cancer cells. Cell 2009; 138:673 - 84; http://dx.doi.org/10.1016/j.cell.2009.06.016; PMID: 19703394
- Di Giammartino DC, Nishida K, Manley JL. Mechanisms and consequences of alternative polyadenylation. Mol Cell 2011; 43:853 - 66; http://dx.doi.org/10.1016/j.molcel.2011.08.017; PMID: 21925375
- Ji Z, Tian B. Reprogramming of 3′ untranslated regions of mRNAs by alternative polyadenylation in generation of pluripotent stem cells from different cell types. PLoS One 2009; 4:e8419; http://dx.doi.org/10.1371/journal.pone.0008419; PMID: 20037631
- To KK, Zhan Z, Litman T, Bates SE. Regulation of ABCG2 expression at the 3′ untranslated region of its mRNA through modulation of transcript stability and protein translation by a putative microRNA in the S1 colon cancer cell line. Mol Cell Biol 2008; 28:5147 - 61; http://dx.doi.org/10.1128/MCB.00331-08; PMID: 18573883
- Newman MA, Hammond SM. Emerging paradigms of regulated microRNA processing. Genes Dev 2010; 24:1086 - 92; http://dx.doi.org/10.1101/gad.1919710; PMID: 20516194
- Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 2009; 10:126 - 39; http://dx.doi.org/10.1038/nrm2632; PMID: 19165215
- Gregory RI, Shiekhattar R. MicroRNA biogenesis and cancer. Cancer Res 2005; 65:3509 - 12; http://dx.doi.org/10.1158/0008-5472.CAN-05-0298; PMID: 15867338
- Carthew RW, Sontheimer EJ. Origins and Mechanisms of miRNAs and siRNAs. Cell 2009; 136:642 - 55; http://dx.doi.org/10.1016/j.cell.2009.01.035; PMID: 19239886
- Winter J, Diederichs S. MicroRNA biogenesis and cancer. Methods Mol Biol 2011; 676:3 - 22; http://dx.doi.org/10.1007/978-1-60761-863-8_1; PMID: 20931386
- Michlewski G, Cáceres JF. Antagonistic role of hnRNP A1 and KSRP in the regulation of let-7a biogenesis. Nat Struct Mol Biol 2010; 17:1011 - 8; http://dx.doi.org/10.1038/nsmb.1874; PMID: 20639884
- Trabucchi M, Briata P, Garcia-Mayoral M, Haase AD, Filipowicz W, Ramos A, et al. The RNA-binding protein KSRP promotes the biogenesis of a subset of microRNAs. Nature 2009; 459:1010 - 4; http://dx.doi.org/10.1038/nature08025; PMID: 19458619
- Van Wynsberghe PM, Kai ZS, Massirer KB, Burton VH, Yeo GW, Pasquinelli AE. LIN-28 co-transcriptionally binds primary let-7 to regulate miRNA maturation in Caenorhabditis elegans. Nat Struct Mol Biol 2011; 18:302 - 8; http://dx.doi.org/10.1038/nsmb.1986; PMID: 21297634
- Arasu P, Wightman B, Ruvkun G. Temporal regulation of lin-14 by the antagonistic action of two other heterochronic genes, lin-4 and lin-28. Genes Dev 1991; 5:1825 - 33; http://dx.doi.org/10.1101/gad.5.10.1825; PMID: 1916265
- Newman MA, Hammond SM. Lin-28: an early embryonic sentinel that blocks Let-7 biogenesis. Int J Biochem Cell Biol 2010; 42:1330 - 3; http://dx.doi.org/10.1016/j.biocel.2009.02.023; PMID: 20619222
- Hagan JP, Piskounova E, Gregory RI. Lin28 recruits the TUTase Zcchc11 to inhibit let-7 maturation in mouse embryonic stem cells. Nat Struct Mol Biol 2009; 16:1021 - 5; http://dx.doi.org/10.1038/nsmb.1676; PMID: 19713958
- Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science 2008; 320:97 - 100; http://dx.doi.org/10.1126/science.1154040; PMID: 18292307
- Lehrbach NJ, Armisen J, Lightfoot HL, Murfitt KJ, Bugaut A, Balasubramanian S, et al. LIN-28 and the poly(U) polymerase PUP-2 regulate let-7 microRNA processing in Caenorhabditis elegans. Nat Struct Mol Biol 2009; 16:1016 - 20; http://dx.doi.org/10.1038/nsmb.1675; PMID: 19713957
- Chang TC, Zeitels LR, Hwang HW, Chivukula RR, Wentzel EA, Dews M, et al. Lin-28B transactivation is necessary for Myc-mediated let-7 repression and proliferation. Proc Natl Acad Sci U S A 2009; 106:3384 - 9; http://dx.doi.org/10.1073/pnas.0808300106; PMID: 19211792
- Heo I, Joo C, Cho J, Ha M, Han J, Kim VN. Lin28 mediates the terminal uridylation of let-7 precursor MicroRNA. Mol Cell 2008; 32:276 - 84; http://dx.doi.org/10.1016/j.molcel.2008.09.014; PMID: 18951094
- Piskounova E, Polytarchou C, Thornton JE, LaPierre RJ, Pothoulakis C, Hagan JP, et al. Lin28A and Lin28B inhibit let-7 microRNA biogenesis by distinct mechanisms. Cell 2011; 147:1066 - 79; http://dx.doi.org/10.1016/j.cell.2011.10.039; PMID: 22118463
- Lee JY, Moon HJ, Lee WK, Chun HJ, Han CW, Jeon YW, et al. Merlin facilitates ubiquitination and degradation of transactivation-responsive RNA-binding protein. Oncogene 2006; 25:1143 - 52; http://dx.doi.org/10.1038/sj.onc.1209150; PMID: 16247459
- Kim YK, Yeo J, Ha M, Kim B, Kim VN. Cell adhesion-dependent control of microRNA decay. Mol Cell 2011; 43:1005 - 14; http://dx.doi.org/10.1016/j.molcel.2011.07.031; PMID: 21925388
- Rissland OS, Hong SJ, Bartel DP. MicroRNA destabilization enables dynamic regulation of the miR-16 family in response to cell-cycle changes. Mol Cell 2011; 43:993 - 1004; http://dx.doi.org/10.1016/j.molcel.2011.08.021; PMID: 21925387
- Gruber JJ, Zatechka DS, Sabin LR, Yong J, Lum JJ, Kong M, et al. Ars2 links the nuclear cap-binding complex to RNA interference and cell proliferation. Cell 2009; 138:328 - 39; http://dx.doi.org/10.1016/j.cell.2009.04.046; PMID: 19632182
- Kumar MS, Pester RE, Chen CY, Lane K, Chin C, Lu J, et al. Dicer1 functions as a haploinsufficient tumor suppressor. Genes Dev 2009; 23:2700 - 4; http://dx.doi.org/10.1101/gad.1848209; PMID: 19903759
- Melo SA, Moutinho C, Ropero S, Calin GA, Rossi S, Spizzo R, et al. A genetic defect in exportin-5 traps precursor microRNAs in the nucleus of cancer cells. Cancer Cell 2010; 18:303 - 15; http://dx.doi.org/10.1016/j.ccr.2010.09.007; PMID: 20951941
- Rotunno M, Zhao Y, Bergen AW, Koshiol J, Burdette L, Rubagotti M, et al. Inherited polymorphisms in the RNA-mediated interference machinery affect microRNA expression and lung cancer survival. Br J Cancer 2010; 103:1870 - 4; http://dx.doi.org/10.1038/sj.bjc.6605976; PMID: 21102586
- Vasudevan S. [Wiley Interdiscip Rev RNA. ] Posttranscriptional Upregulation by MicroRNAs. 2011; 2011
- Tserel L, Runnel T, Kisand K, Pihlap M, Bakhoff L, Kolde R, et al. MicroRNA expression profiles of human blood monocyte-derived dendritic cells and macrophages reveal miR-511 as putative positive regulator of Toll-like receptor 4. J Biol Chem 2011; 286:26487 - 95; http://dx.doi.org/10.1074/jbc.M110.213561; PMID: 21646346
- Mukherji S, Ebert MS, Zheng GX, Tsang JS, Sharp PA, van Oudenaarden A. MicroRNAs can generate thresholds in target gene expression. Nat Genet 2011; 43:854 - 9; http://dx.doi.org/10.1038/ng.905; PMID: 21857679
- Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, et al. Argonaute2 is the catalytic engine of mammalian RNAi. Science 2004; 305:1437 - 41; http://dx.doi.org/10.1126/science.1102513; PMID: 15284456
- Wu L, Fan J, Belasco JG. Importance of translation and nonnucleolytic ago proteins for on-target RNA interference. Curr Biol 2008; 18:1327 - 32; http://dx.doi.org/10.1016/j.cub.2008.07.072; PMID: 18771919
- Giraldez AJ, Mishima Y, Rihel J, Grocock RJ, Van Dongen S, Inoue K, et al. Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. Science 2006; 312:75 - 9; http://dx.doi.org/10.1126/science.1122689; PMID: 16484454
- Wu L, Fan J, Belasco JG. MicroRNAs direct rapid deadenylation of mRNA. Proc Natl Acad Sci U S A 2006; 103:4034 - 9; http://dx.doi.org/10.1073/pnas.0510928103; PMID: 16495412
- Chekulaeva M, Filipowicz W. Mechanisms of miRNA-mediated post-transcriptional regulation in animal cells. Curr Opin Cell Biol 2009; 21:452 - 60; http://dx.doi.org/10.1016/j.ceb.2009.04.009; PMID: 19450959
- Fabian MR, Sundermeier TR, Sonenberg N. Understanding how miRNAs post-transcriptionally regulate gene expression. Prog Mol Subcell Biol 2010; 50:1 - 20; http://dx.doi.org/10.1007/978-3-642-03103-8_1; PMID: 19841878
- Yekta S, Shih IH, Bartel DP. MicroRNA-directed cleavage of HOXB8 mRNA. Science 2004; 304:594 - 6; http://dx.doi.org/10.1126/science.1097434; PMID: 15105502
- Beilharz TH, Humphreys DT, Clancy JL, Thermann R, Martin DI, Hentze MW, et al. microRNA-mediated messenger RNA deadenylation contributes to translational repression in mammalian cells. PLoS One 2009; 4:e6783; http://dx.doi.org/10.1371/journal.pone.0006783; PMID: 19710908
- Eulalio A, Huntzinger E, Nishihara T, Rehwinkel J, Fauser M, Izaurralde E. Deadenylation is a widespread effect of miRNA regulation. RNA 2009; 15:21 - 32; http://dx.doi.org/10.1261/rna.1399509; PMID: 19029310
- Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 2010; 466:835 - 40; http://dx.doi.org/10.1038/nature09267; PMID: 20703300
- Nilsen TW. Mechanisms of microRNA-mediated gene regulation in animal cells. Trends Genet 2007; 23:243 - 9; http://dx.doi.org/10.1016/j.tig.2007.02.011; PMID: 17368621
- Liu J, Rivas FV, Wohlschlegel J, Yates JR 3rd, Parker R, Hannon GJ. A role for the P-body component GW182 in microRNA function. Nat Cell Biol 2005; 7:1261 - 6; http://dx.doi.org/10.1038/ncb1333; PMID: 16284623
- Lian SL, Li S, Abadal GX, Pauley BA, Fritzler MJ, Chan EK. The C-terminal half of human Ago2 binds to multiple GW-rich regions of GW182 and requires GW182 to mediate silencing. RNA 2009; 15:804 - 13; http://dx.doi.org/10.1261/rna.1229409; PMID: 19324964
- Ding XC, Grosshans H. Repression of C. elegans microRNA targets at the initiation level of translation requires GW182 proteins. EMBO J 2009; 28:213 - 22; http://dx.doi.org/10.1038/emboj.2008.275; PMID: 19131968
- Fabian MR, Mathonnet G, Sundermeier T, Mathys H, Zipprich JT, Svitkin YV, et al. Mammalian miRNA RISC recruits CAF1 and PABP to affect PABP-dependent deadenylation. Mol Cell 2009; 35:868 - 80; http://dx.doi.org/10.1016/j.molcel.2009.08.004; PMID: 19716330
- Braun JE, Huntzinger E, Fauser M, Izaurralde E. GW182 proteins directly recruit cytoplasmic deadenylase complexes to miRNA targets. Mol Cell 2011; 44:120 - 33; http://dx.doi.org/10.1016/j.molcel.2011.09.007; PMID: 21981923
- Eulalio A, Huntzinger E, Izaurralde E. GW182 interaction with Argonaute is essential for miRNA-mediated translational repression and mRNA decay. Nat Struct Mol Biol 2008; 15:346 - 53; http://dx.doi.org/10.1038/nsmb.1405; PMID: 18345015
- Chekulaeva M, Mathys H, Zipprich JT, Attig J, Colic M, Parker R, et al. miRNA repression involves GW182-mediated recruitment of CCR4-NOT through conserved W-containing motifs. Nat Struct Mol Biol 2011; 18:1218 - 26; http://dx.doi.org/10.1038/nsmb.2166; PMID: 21984184
- Su H, Meng S, Lu Y, Trombly MI, Chen J, Lin C, et al. Mammalian hyperplastic discs homolog EDD regulates miRNA-mediated gene silencing. Mol Cell 2011; 43:97 - 109; http://dx.doi.org/10.1016/j.molcel.2011.06.013; PMID: 21726813
- Fabian MR, Cieplak MK, Frank F, Morita M, Green J, Srikumar T, et al. miRNA-mediated deadenylation is orchestrated by GW182 through two conserved motifs that interact with CCR4-NOT. Nat Struct Mol Biol 2011; 18:1211 - 7; http://dx.doi.org/10.1038/nsmb.2149; PMID: 21984185
- Yang Z, Jakymiw A, Wood MR, Eystathioy T, Rubin RL, Fritzler MJ, et al. GW182 is critical for the stability of GW bodies expressed during the cell cycle and cell proliferation. J Cell Sci 2004; 117:5567 - 78; http://dx.doi.org/10.1242/jcs.01477; PMID: 15494374
- Jakymiw A, Lian S, Eystathioy T, Li S, Satoh M, Hamel JC, et al. Disruption of GW bodies impairs mammalian RNA interference. Nat Cell Biol 2005; 7:1267 - 74; http://dx.doi.org/10.1038/ncb1334; PMID: 16284622
- Lian S, Jakymiw A, Eystathioy T, Hamel JC, Fritzler MJ, Chan EK. GW bodies, microRNAs and the cell cycle. Cell Cycle 2006; 5:242 - 5; http://dx.doi.org/10.4161/cc.5.3.2410; PMID: 16418578
- Flemr M, Ma J, Schultz RM, Svoboda P. P-body loss is concomitant with formation of a messenger RNA storage domain in mouse oocytes. Biol Reprod 2010; 82:1008 - 17; http://dx.doi.org/10.1095/biolreprod.109.082057; PMID: 20075394
- Eulalio A, Behm-Ansmant I, Schweizer D, Izaurralde E. P-body formation is a consequence, not the cause, of RNA-mediated gene silencing. Mol Cell Biol 2007; 27:3970 - 81; http://dx.doi.org/10.1128/MCB.00128-07; PMID: 17403906
- Spassov DS, Jurecic R. The PUF family of RNA-binding proteins: does evolutionarily conserved structure equal conserved function?. IUBMB Life 2003; 55:359 - 66; http://dx.doi.org/10.1080/15216540310001603093; PMID: 14584586
- Miles WO, Tschöp K, Herr A, Ji JY, Dyson NJ. Pumilio facilitates miRNA regulation of the E2F3 oncogene. Genes Dev 2012; 26:356 - 68; http://dx.doi.org/10.1101/gad.182568.111; PMID: 22345517
- Lin CC, Liu LZ, Addison JB, Wonderlin WF, Ivanov AV, Ruppert JM. A KLF4-miRNA-206 autoregulatory feedback loop can promote or inhibit protein translation depending upon cell context. Mol Cell Biol 2011; 31:2513 - 27; http://dx.doi.org/10.1128/MCB.01189-10; PMID: 21518959
- Liu Q, Fu H, Sun F, Zhang H, Tie Y, Zhu J, et al. miR-16 family induces cell cycle arrest by regulating multiple cell cycle genes. Nucleic Acids Res 2008; 36:5391 - 404; http://dx.doi.org/10.1093/nar/gkn522; PMID: 18701644
- Garnon J, Lachance C, Di Marco S, Hel Z, Marion D, Ruiz MC, et al. Fragile X-related protein FXR1P regulates proinflammatory cytokine tumor necrosis factor expression at the post-transcriptional level. J Biol Chem 2005; 280:5750 - 63; http://dx.doi.org/10.1074/jbc.M401988200; PMID: 15548538
- Hornstein E, Tang H, Meyuhas O. Mitogenic and nutritional signals are transduced into translational efficiency of TOP mRNAs. Cold Spring Harb Symp Quant Biol 2001; 66:477 - 84; http://dx.doi.org/10.1101/sqb.2001.66.477; PMID: 12762050
- Ørom UA, Nielsen FC, Lund AH. MicroRNA-10a binds the 5’UTR of ribosomal protein mRNAs and enhances their translation. Mol Cell 2008; 30:460 - 71; http://dx.doi.org/10.1016/j.molcel.2008.05.001; PMID: 18498749
- Till S, Lejeune E, Thermann R, Bortfeld M, Hothorn M, Enderle D, et al. A conserved motif in Argonaute-interacting proteins mediates functional interactions through the Argonaute PIWI domain. Nat Struct Mol Biol 2007; 14:897 - 903; http://dx.doi.org/10.1038/nsmb1302; PMID: 17891150
- Iwasaki S, Tomari Y. Argonaute-mediated translational repression (and activation). Fly (Austin) 2009; 3:204 - 6; PMID: 19556851
- Siomi MC, Zhang Y, Siomi H, Dreyfuss G. Specific sequences in the fragile X syndrome protein FMR1 and the FXR proteins mediate their binding to 60S ribosomal subunits and the interactions among them. Mol Cell Biol 1996; 16:3825 - 32; PMID: 8668200
- Seal R, Temperley R, Wilusz J, Lightowlers RN, Chrzanowska-Lightowlers ZM. Serum-deprivation stimulates cap-binding by PARN at the expense of eIF4E, consistent with the observed decrease in mRNA stability. Nucleic Acids Res 2005; 33:376 - 87; http://dx.doi.org/10.1093/nar/gki169; PMID: 15653638
- Roy AL, Chakrabarti D, Datta B, Hileman RE, Gupta NK. Natural mRNA is required for directing Met-tRNA(f) binding to 40S ribosomal subunits in animal cells: involvement of Co-eIF-2A in natural mRNA-directed initiation complex formation. Biochemistry 1988; 27:8203 - 9; http://dx.doi.org/10.1021/bi00421a033; PMID: 3233204
- Unhavaithaya Y, Hao Y, Beyret E, Yin H, Kuramochi-Miyagawa S, Nakano T, et al. MILI, a PIWI-interacting RNA-binding protein, is required for germ line stem cell self-renewal and appears to positively regulate translation. J Biol Chem 2009; 284:6507 - 19; http://dx.doi.org/10.1074/jbc.M809104200; PMID: 19114715
- Jackson RJ, Hellen CU, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol 2010; 11:113 - 27; http://dx.doi.org/10.1038/nrm2838; PMID: 20094052
- Hinnebusch AG. eIF3: a versatile scaffold for translation initiation complexes. Trends Biochem Sci 2006; 31:553 - 62; http://dx.doi.org/10.1016/j.tibs.2006.08.005; PMID: 16920360
- Diederichs S, Jung S, Rothenberg SM, Smolen GA, Mlody BG, Haber DA. Coexpression of Argonaute-2 enhances RNA interference toward perfect match binding sites. Proc Natl Acad Sci U S A 2008; 105:9284 - 9; http://dx.doi.org/10.1073/pnas.0800803105; PMID: 18591665
- Eiring AM, Harb JG, Neviani P, Garton C, Oaks JJ, Spizzo R, et al. miR-328 functions as an RNA decoy to modulate hnRNP E2 regulation of mRNA translation in leukemic blasts. Cell 2010; 140:652 - 65; http://dx.doi.org/10.1016/j.cell.2010.01.007; PMID: 20211135
- Leung AK, Vyas S, Rood JE, Bhutkar A, Sharp PA, Chang P. Poly(ADP-ribose) regulates stress responses and microRNA activity in the cytoplasm. Mol Cell 2011; 42:489 - 99; http://dx.doi.org/10.1016/j.molcel.2011.04.015; PMID: 21596313
- Poliseno L, Salmena L, Zhang J, Carver B, Haveman WJ, Pandolfi PP. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 2010; 465:1033 - 8; http://dx.doi.org/10.1038/nature09144; PMID: 20577206
- Cazalla D, Yario T, Steitz JA. Down-regulation of a host microRNA by a Herpesvirus saimiri noncoding RNA. Science 2010; 328:1563 - 6; http://dx.doi.org/10.1126/science.1187197; PMID: 20558719
- Ebert MS, Sharp PA. Emerging roles for natural microRNA sponges. Curr Biol 2010; 20:R858 - 61; http://dx.doi.org/10.1016/j.cub.2010.08.052; PMID: 20937476
- Meisner NC, Filipowicz W. Properties of the regulatory RNA-binding protein HuR and its role in controlling miRNA repression. Adv Exp Med Biol 2010; 700:106 - 23; http://dx.doi.org/10.1007/978-1-4419-7823-3_10; PMID: 21627034
- Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell 2006; 125:1111 - 24; http://dx.doi.org/10.1016/j.cell.2006.04.031; PMID: 16777601
- Srikantan S, Abdelmohsen K, Lee EK, Tominaga K, Subaran SS, Kuwano Y, et al. Translational control of TOP2A influences doxorubicin efficacy. Mol Cell Biol 2011; 31:3790 - 801; http://dx.doi.org/10.1128/MCB.05639-11; PMID: 21768308
- Murphy AJ, Guyre PM, Pioli PA. Estradiol suppresses NF-kappa B activation through coordinated regulation of let-7a and miR-125b in primary human macrophages. J Immunol 2010; 184:5029 - 37; http://dx.doi.org/10.4049/jimmunol.0903463; PMID: 20351193
- Leung AK, Calabrese JM, Sharp PA. Quantitative analysis of Argonaute protein reveals microRNA-dependent localization to stress granules. Proc Natl Acad Sci U S A 2006; 103:18125 - 30; http://dx.doi.org/10.1073/pnas.0608845103; PMID: 17116888
- Wyman SK, Knouf EC, Parkin RK, Fritz BR, Lin DW, Dennis LM, et al. Post-transcriptional generation of miRNA variants by multiple nucleotidyl transferases contributes to miRNA transcriptome complexity. Genome Res 2011; 21:1450 - 61; http://dx.doi.org/10.1101/gr.118059.110; PMID: 21813625
- Li J, Yang Z, Yu B, Liu J, Chen X. Methylation protects miRNAs and siRNAs from a 3′-end uridylation activity in Arabidopsis. Curr Biol 2005; 15:1501 - 7; http://dx.doi.org/10.1016/j.cub.2005.07.029; PMID: 16111943
- Houbaviy HB, Murray MF, Sharp PA. Embryonic stem cell-specific MicroRNAs. Dev Cell 2003; 5:351 - 8; http://dx.doi.org/10.1016/S1534-5807(03)00227-2; PMID: 12919684
- Newman MA, Mani V, Hammond SM. Deep sequencing of microRNA precursors reveals extensive 3′ end modification. RNA 2011; 17:1795 - 803; http://dx.doi.org/10.1261/rna.2713611; PMID: 21849429
- Burns DM, D’Ambrogio A, Nottrott S, Richter JD. CPEB and two poly(A) polymerases control miR-122 stability and p53 mRNA translation. Nature 2011; 473:105 - 8; http://dx.doi.org/10.1038/nature09908; PMID: 21478871
- Katoh T, Sakaguchi Y, Miyauchi K, Suzuki T, Kashiwabara S, Baba T, et al. Selective stabilization of mammalian microRNAs by 3′ adenylation mediated by the cytoplasmic poly(A) polymerase GLD-2. Genes Dev 2009; 23:433 - 8; http://dx.doi.org/10.1101/gad.1761509; PMID: 19240131
- Jones MR, Quinton LJ, Blahna MT, Neilson JR, Fu S, Ivanov AR, et al. Zcchc11-dependent uridylation of microRNA directs cytokine expression. Nat Cell Biol 2009; 11:1157 - 63; http://dx.doi.org/10.1038/ncb1931; PMID: 19701194