Abstract
It has been well-documented that nuclear processing of primary transcripts of RNA polymerase II occurs co-transcriptionally and is functionally coupled to transcription. Moreover, increasing evidence indicates that transcription influences pre-mRNA splicing and even several post-splicing RNA processing events. In this review, we discuss the issues of how RNA polymerase II modulates co-transcriptional RNA processing events via its carboxyl terminal domain, and the protein domains involved in coupling of transcription and RNA processing events. In addition, we describe how transcription influences the expression or stability of mRNAs through the formation of distinct mRNP complexes. Finally, we delineate emerging findings that chromatin modifications function in the regulation of RNA processing steps, especially splicing, in addition to transcription. Overall, we provide a comprehensive view that transcription could integrate different control systems, from epigenetic to post-transcriptional control, for efficient gene expression.
Introduction
In eukaryotes, RNA polymerase II (pol II) catalyzes the synthesis of mRNAs (mRNAs), long non-coding RNAs and several different types of small RNAs, such as the spliceosomal small nuclear RNAs and microRNAs. The primary transcripts of these RNAs are immediately bound by ribonucleoproteins (RNPs) and processed into mature and functional forms via several distinct but coordinated steps.
The vast majority of precursor mRNAs (pre-mRNAs) undergo 5′ capping, splicing and 3′ polyadenylation before export to the cytoplasm for subsequent translation on ribosomes. Although each of these pre-mRNA processing steps can be recaptured independently in vitro, a number of studies have indicated that all these steps are integrated with each other and coupled to transcription.Citation1 Indeed, trans-acting factors involved in RNA processing can be recruited to emerging transcripts at different stages of transcription. Here, we discuss our current understanding of the molecular connection between transcription and pre-mRNA processing and reciprocal influences between different RNA biogenesis steps. Our discussion also includes recent evidence that nucleosome structure and DNA methylation constitute an additional means by which pre-mRNA splicing may be regulated.
Coordination of transcription and RNA processing by the RNA polymerase II CTD
RNA pol II is a large protein complex. The yeast RNA pol II consists of a 10-subunit core complex and the heterodimeric subcomplex Rbp4/Rbp7.Citation2 The catalytic subunit Rbp1 contains an unstructured carboxyl-terminal domain (CTD) that is not present in its functional analogs in RNA pol I and pol III. The pol II CTD consists of heptapeptide (YSPTSPS) repeats, the number of which varies from 26 in Saccharomyces cerevisiae to 52 in mammals.Citation3 These repeats undergo several different post-translational modifications; most essential is phosphorylation of tyrosine (Tyr1), serine (Ser2, Ser5 and Ser7) and threonine (Thr4) residues (). Moreover, some lysine and arginine residues in certain non-consensus repeats are subject to modifications such as acetylation, methylation, ubiquitylation and sumoylation. The dynamic change of the phosphorylation status of the CTD correlates well with the progression of transcription. Reversible phosphorylation involves several specific kinases and phosphatases.Citation3 At the transcription initiation stage, CDK7, a component of the general transcriptional factor TFIIH, phosphorylates Ser5 and perhaps Ser7 of the CTD.Citation4,Citation5 Subsequently, the elongation factor P-TEFb (CDK9/cyclin T) phosphorylates Ser2 while Ser5 is then dephosphorylated by several different phosphatases (for a review, see ref. Citation6). Most notably, the sequential phosphorylation and dephosphorylation of Ser5 and Ser2 is important not only for transcription but also for co-transcriptional processing of pre-mRNAs (for a review, see ref. Citation3 and Citation7). In addition, cellular signaling kinases such as ERK can also phosphorylate specific serine residues of the CTD and may thereby affect transcription and/or RNA processing.Citation8
Figure 1. The C-terminal domain of RNA pol II coordinates nuclear pre-mRNA processing. (A) Differential phosphorylation of the pol II CTD during transcription. The pol II CTD coordinates 5′ capping (B), 3′-end polyadenylation (C), transcription termination of short transcripts (D) and spliceosome assembly and splicing activation (E). All abbreviations are defined in the text.
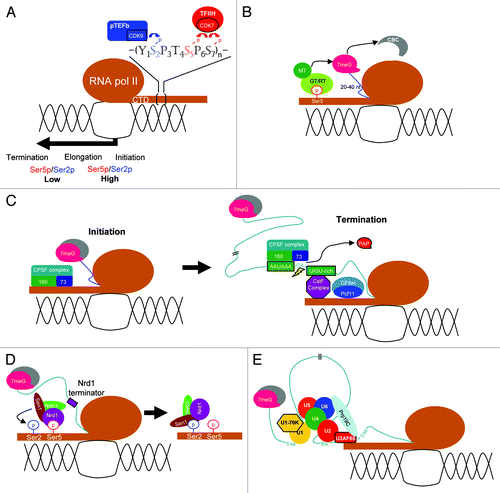
CTD and 5′ capping
The 5′ end of each RNA pol II transcript acquires the unique 7-methylguanosine cap structure.Citation9 Capping occurs coincidently with promoter-proximal pausing of pol II, which is mediated by 5,6-dichloro-1-β-D-ribofuranosylbenzimidazole (DRB) sensitivity-inducing factor (DSIF) and the negative elongation factor (NELF), while the nascent transcript of ~20–40 nucleotides is being generated.Citation10 The formation of the 5′ cap requires three enzymatic activities, including RNA 5′-triphosphatase (RT), guanylyltransferase (GT) and methyltransferase.Citation9,Citation10 The RT removes the 5′-end γ-phosphate of the nascent transcript to generate a diphosphate-ended RNA. Subsequently, GT transfers a guanosine monophosphate to the 5′ diphosphate terminus of the RNA, followed by guanine methylation by methyltransferase. The capping enzyme is a heterodimer of the RT (Cet1) and GT (Ceg1) subunits in yeast, whereas it is a bi-functional single protein with both RT and GT activities in higher eukaryotes.Citation11,Citation12 Nevertheless, both mammalian and yeast GT domains preferentially recognize CTD phosphorylated at Ser5, which leads to the allosteric activation of GT activity ().Citation11,Citation13 In addition to Ser5 phosphorylation, the Spt5 subunit of DSIF also interacts with the capping enzyme and activates mRNA capping.Citation14,Citation15 After cap formation, P-TEFb phosphorylates Ser2 of the CTD as well as DSIF and NELF;Citation16,Citation17 the dissociation of DSIF and NELF releases the promoter-proximal pausing of pol II. Interestingly, fission yeast P-TEFb also interacts with the cap-methyltransferase Pcm1 and may thus facilitate the completion of the capping process.Citation18 Therefore, capping can serve as a gatekeeper for the productive elongation stage of transcription. Thus, pre-mRNA capping and transcription are tightly coupled and influence each other.
CTD and 3′-end processing
Pre-mRNA 3′-end processing also shows strong interdependence with the pol II CTD. The vast majority of mRNAs contain a non-templated poly(A) tail. In metazoans, polyadenylation involves several trans-acting protein complexes and corresponding cis-acting RNA elements (for a review, see refs. Citation19 and Citation20) Initially, the polyadenylation signal AAUAAA and its downstream GU-rich element are recognized by the cleavage/polyadenylation-specificity factor (CPSF) and cleavage stimulation factor (CstF), respectively. The CPSF-73 subunit of CPSF is responsible for cleaving the mRNA at the CA dinucleotide located ~10–30 nucleotides downstream of the polyadenylation signal prior to polyadenylation catalyzed by poly(A) polymerase.Citation21 During transcription initiation, CPSF is brought to the pre-initiation complex by TFIID, and it remains associated with pol II during elongation ().Citation22 In mammalian cells, RNAs transcribed by CTD-truncated pol II are not efficiently polyadenylated, indicating the importance of CTD in polyadenylation.Citation23 In addition, the finding that RNA pol II containing Ser2-phosphorylated CTD predominates at the 3′ end of genes during transcription suggests that Ser2 phosphorylation plays a regulatory role in 3′-end pre-mRNA processing.Citation24 Coincidently, deletion of the yeast Ser2 kinase Ctk1 impairs polyadenylation without obvious effects on transcription elongation.Citation25 However, unlike capping enzymes, most of the 3′-end processing factors have no strong preference for specific phospho-isoforms of the CTD.Citation25
For certain histones required for DNA replication, their pre-mRNAs undergo a unique endonucleolytic cleavage reaction at the 3′ end instead of polyadenylation. This cleavage requires the stem-loop binding protein SLBP and the U7 small nuclear RNP (snRNP) that bind to corresponding cis-elements on histone pre-mRNAs.Citation19 A recent study showed that CDK9-mediated phosphorylation of CTD Thr4 may facilitate the recruitment of SLBP and CPSF-100, a factor common to both histone mRNA processing and general mRNA polyadenylation, to promote histone mRNA maturation.Citation26 Nevertheless, whether this CTD phosphorylation is modulated by the cell cycle remains an interesting question.
CTD and transcription termination
A large amount of evidence from yeast studies has indicated that the interaction between the pol II CTD and the polyadenylation machinery is important for transcription termination. The Pcf11 subunit of the yeast cleavage/polyadenylation factor binds to the CTD phosphorylated on Ser2 via its CTD-interacting domain.Citation1,Citation27 Notably, a recent report shows that Tyr1 phosphorylation of the pol II CTD excludes Pcf11 during elongation and thereby suppresses premature termination.Citation28 However, the decline of Tyr1 phosphroylation prior to the polyadenylation site enables phsopho-Ser2 CTD to recruit Pcf11 and other termination factors. Pcf11 mediates transcription termination through its cooperation with another CTD-interacting domain-containing factor, Rtt103 and the 5′–3′ exonuclease Rat1, which facilitates release of the transcription elongation complex from the transcript.Citation29,Citation30 Another CTD-interacting domain-containing RNA-binding protein, Nrd1, also contributes to transcription termination.Citation31 In contrast to Pcf11 and Rtt103, Nrd1 preferentially interacts with the CTD phosphorylated on Ser5; this interaction requires the cis conformation of the upstream phospho-Ser5-Pro6 peptidyl-prolyl bond of the CTD.Citation32 Nrd1 forms a complex with Nab3 and Sen1 to participate in transcription termination and 3′-end processing of certain non-coding RNAs ().Citation33 The Nrd1/Nab3/Sen1 complex initially associates with pol II at the promoter region where phospho-Ser5 CTD is predominant and then moves along the gene with pol II toward the 3′ end. A recent report showed that disruption of the Sen1-Ser2 CTD interaction prevents transcription termination.Citation34 Therefore, the Nrd1/Nab3/Sen1 complex may be handed over from phospho-Ser5 to phospho-Ser2 via a sequential interaction of Nrd1 and Sen1 with the CTD. Interestingly, the Nrd1-CTD interaction is also impaired by phospho-Tyr1, suggesting that different termination decisions may depend on phospho-Tyr1.Citation28 Taken together, all evidence has suggested that sequential phosphorylation of the CTD is likely important for proper processing of pre-mRNAs as well as transcription elongation and termination.
CTD and pre-mRNA splicing
Pre-mRNA splicing is catalyzed by the spliceosome, a large RNP complex composed of five spliceosomal small nuclear RNAs and nearly 200 proteins, including members of the SR protein family.Citation35 SR proteins typically contain RNA-binding motifs for cis-element recognition and one or more arginine-serine dipeptide-rich (RS) domains essentially for protein-protein interactions.Citation36 Yeast two-hybrid experiments have unveiled interactions between the pol II CTD and SR proteins, suggesting that pol II recruits SR proteins to nascent transcripts.Citation37 Accordingly, subnuclear localization of SR proteins is profoundly influenced by pol II activity (for a review, see ref. Citation38), which suggests that splicing is coupled to transcription in cells.Citation38,Citation39 Moreover, mass spectrometry analysis of pol II-associating proteins identified a number of SR proteins as well as the U1 snRNP.Citation40 Recently, an in vitro functional assay revealed that RNA pol II also interacts with the splicing factor U2AF65 and the multiprotein PRP19 complex via its CTD to promote splicing ().Citation41 The PRP19 complex joins the spliceosome only at the late stage of splicing and activates the catalytic reaction, suggesting that the CTD may also help to coordinate spliceosome activation.Citation41 Collectively, these results suggest a recruitment model in which pol II affects pre-mRNA splicing via the interaction of the CTD with splicing factors.Citation42 In fact, the impact of transcription elongation on pre-mRNA splicing has been unveiled for a decade. An early report showed that a mutant Drosophila RNA pol II with decreased elongation activity influences alternative splicing of reporter transcripts.Citation43 More lines of evidence then indicated that the RNA pol II processivity affects splicing, suggesting kinetic coupling between transcription and splicing (for a review, see refs. Citation44 and Citation45). Recent high-throughput analysis provides a comprehensive view on the impact of pol II elongation on splicing. Reduced elongation rate causes higher levels of pol II occupancy in introns.Citation46 Moreover, pol II often accumulates or stalls in the introns that have suboptimal downstream splice sites, and thereby causes regulated exon inclusion.Citation46 Therefore, it is assumed that a reduced elongation rate may provide ample time for splicing factors to recognize weak splice signals.
Physiological significance of transcription rate-modulated splicing regulation has also been revealed. It is noteworthy that pol II elongation-dependent splicing regulation is particularly involved in the expression of a set of RNA processing factors in response to changes of growth conditions, which then downregulates those factors via nonsense-mediated mRNA decay (NMD; see below) or causes their exon inclusion.Citation46 Conceivably, such a splicing change has a profound effect on the transcriptome. A more detailed mechanism has been shown on the alternative splicing of proapoptotic Bcl-x and caspase 9 caused by UV-induced DNA damage, which acts via hyperphosphorylation of both Ser2 and Ser5 of RNA pol II and, thus, inhibits pol II elongation.Citation47 A more recent report shows that a pausing site embedded within the exonic cis-regulatory element of Bcl-x slows down the elongating pol II and, hence, a putative repressive complex forms. Overexpression of the elongation regulator 1 TCERG1/CA150, which accelerates pol II elongation, reactivates the suppressed 5′ splice site of Bcl-x.Citation48 Another recent report showing that a mRNP assembled on a large set of regulated exons embedded in A/T-rich DNA can interact with pol II and modulates transcription elongation, also indicates the impact of chromatin-induced transcription pausing on alternative splicing.Citation49
So far, we discuss how transcription elongation affects splicing. However, certain aspects of pre-mRNA splicing may influence the rate of transcription elongation, especially on the activity of P-TEFb. One report showed that depletion of the SR protein SRSF2/SC35 impairs P-TEFb recruitment to the pol II elongating complex and subsequent phosphorylation of CTD-Ser2 and, thus, attenuates elongation.Citation50 Another finding showed that the 7SK snRNP, which inhibits P-TEFb kinase activity, modulates not only transcription elongation but also alternative splicing.Citation51 Therefore, these observations infer that transcription elongation and alternative splicing are coupled, and that regulation of one process affects the rate of the other.
Protein domains that mediate the link between transcription and splicing
The interconnection between the transcription machinery, essentially RNA pol II, and different pre-mRNA processing factors, contributes substantially to the coupling of transcription and splicing events. For example, the multifunctional protein p54nrb forms a heterodimer with the splicing factor PSF to interact with the pol II CTD, and such an interaction has been implicated in early spliceosome assembly during transcription.Citation52 In addition, p54nrb interacts specifically with the transcription factor Sox9 and modulates both the promoter activity and alternative splicing of Sox9 targets.Citation53 Thus, proteins with dual roles in gene-specific transcription and pre-mRNA processing may function in coordinating gene expression. Three domains, i.e. the RS as well as the WW and FF domains (see below), are most representative in splicing factors that associate with pol II. In this section, we discuss the common domains of splicing factors implicated in linking transcription with pre-mRNA splicing.
RS domain
The involvement of the RS domain in pre-mRNA splicing is firmly established (for a review, see ref. Citation54 and Citation55). The canonical SR splicing factors contain a typical RS domain that consists of successive RS dipeptides. In addition, SR-like splicing factors, such as U1-70K and SRm160, contain degenerate RS domains, in which dispersed RS or arginine-aspartate/glutamate dipeptides exist. Differential phosphorylation of the RS domain by the SRPK and Clk/Sty families of kinases modulates the cellular localization and activity of SR proteins.Citation54 Moreover, in vitro studies have demonstrated that dephosphorylation of SR proteins is also important for splicing.Citation56 The spliceosome-associated protein phosphatase 2Cγ and the activity of protein phosphatase 1/2A are required for early spliceosome assembly and the catalytic reaction of splicing, respectively,Citation57,Citation58 but whether these phosphatases are specific to SR splicing factors remains to be clarified. In addition to splicing, a set of SR proteins also functions in mRNA export; these proteins include the yeast SR-like Npl3 protein and several mammalian shuttling SR proteins.Citation59,Citation60 Nuclear export of Npl3 is facilitated by arginine methylation of its atypical RS domain by Hmt1 and dephosphorylation by the phosphatase Glc7.Citation61,Citation62 Analogously, mammalian SR shuttling proteins also undergo dephosphorylation during export, and dephosphorylated SR proteins are engaged in mRNA translation.Citation63-Citation65 These observations further imply that dynamic phosphorylation of SR proteins, from transcription through translation, governs their activities in controlling gene expression.
Physical interactions between several prototypical SR proteins and phosphorylated pol II CTD support the function of SR proteins in coordinating pre-mRNA splicing and transcription as discussed above.Citation40 In general, SR proteins facilitate exon definition and intron bridging during spliceosome assembly, essentially through RS domain-mediated protein-protein interactions.Citation54 Many SR proteins also participate in alternative splice site choice by specific recognition of regulatory cis-elements, namely exonic or intronic splicing enhancers or silencers.Citation55 Interestingly, in vitro studies have shown that the CTD can substitute for the RS domain of an SR protein to promote early spliceosome assembly, further indicating the significance of recruiting SR proteins to pol II during transcription elongation for subsequent splicing activation.Citation66
RS domains were originally thought to bind RNA non-specifically due to their high content of positively charged residues. Experimental evidence has then indicated that the RS domain can promote or stabilize base-pairing of U1 and U2 snRNAs with the 5′ splice site and branch site of introns perhaps through its direct contact with short snRNA-pre-mRNA helices.Citation67,Citation68 Therefore, SR proteins that are recruited by the RNA pol II CTD may facilitate early spliceosome assembly not only through interaction with proteins but also with RNAs.
WW and FF domain
The WW and FF domains, named for their two spaced signature tryptophan and phenylalanine residues, respectively, also represent protein interaction modules.Citation69,Citation70 The WW domain is composed of ~40 amino acid residues and folds as a triple-stranded sheet that binds to proline-rich peptides.Citation70 WW domain subgroups are distinguished by their similar but slightly distinct ligands. For example, group I and II recognize the PPxY (x denotes variable residues) and PPLP motifs, respectively. The WW domain exists in a large variety of proteins, including several transcriptional co-activators and spliceosomal components.Citation70 The FF domain often co-exists with the WW domain in those proteins. The FF domain contains ~50–60 residues and folds into a three-α-helix structure. The first α-helix contains a highly conserved FxxL motif, which can be specifically recognized by nuclear receptors, suggesting that FF domain-containing proteins may be responsible for ligand-dependent transcriptional activation.Citation69
Several proteins containing a WW or FF domain have been found to link transcription and splicing by their interaction with pol II and splicing factors. The yeast WW domain-containing splicing factor Prp40 interacts directly with the phosphorylated pol II CTD via its WW domain.Citation71 Prp40 associates with the U1 snRNP and interacts with the U5-associated protein Prp8 and the branch point-binding protein (yeast BBP; human SF1).Citation72,Citation73 Thus, Prp40 may mediate intron bridging early during spliceosome assembly. It is notable that the human orthologs of Prp40, i.e., FBP11 and FBP21, also interact with the phosphorylated CTD and associate with the 17S U2 snRNP.Citation74 Importantly, both the Prp40-BBP and FBP11/21-SF1 interactions engage their respective WW domain and proline-rich motif, indicating that the interactions among these splicing factors are evolutionarily conserved.Citation73,Citation75,Citation76 Moreover, early reports have shown that the WW and FF domain-containing TCERG1 interacts with the phosphorylated CTD and the splicing factors that associate with the 3′ end of the intron and that TCERG1 can activate splicing via its WW and FF domains.Citation77,Citation78 More recent evidence indicates that TCERG1 regulates alternative splicing via modulating pol II transcription rateCitation48 (see above) and, thus, supports previous findings. Therefore, yeast WW-containing proteins may promote early spliceosome formation, whereas mammalian analogs have an additional function in alternative splicing.
Co-transcriptional assembly of mRNA-containing ribonucleoproteins
RNA binding proteins can be loaded onto a nascent transcript when it just begins to emerge from RNA pol II but function in following nuclear RNA processing steps. Some of the proteins may remain associated with mature mRNAs and participate in post-splicing events such as mRNA export, stability control and translation. We discuss two co-transcriptionally loaded protein complexes, namely the transcription/export (TREX) complex and exon-junction complex (EJC), which escort mRNAs to the cytoplasm and how these complexes functionally connect different steps of RNA biogenesis.
The TREX complex links transcription and mRNA export
The evolutionarily conserved TREX complex is composed of the THO complex, which functions in transcription elongation, and the mRNA export factors UAP56 (Sub2 in yeast) and Aly/REF (Yra1 in yeast). The yeast THO complex contains four subunits (Tho2, Hpr1, Mft1 and Thp2), whereas the mammalian complex contains only two yeast-equivalent THO components (Tho2 and Hpr1) and three other proteins.Citation79 Although a recent study showed that the PRP19 splicing complex could recruit the TREX complex to actively transcribed genes,Citation80 it is generally believed that yeast THO is recruited to activated genes by RNA pol II.Citation81 Chromatin immunoprecipitation has revealed that THO shows biased localization toward the 3′ end of genes and interacts with the 3′-end processing factor Pcf11.Citation82,Citation83 These results suggest that THO also participates in 3′-end processing and/or transcription termination.Citation84 Pcf11 recruits Yra1 to THO. The mutually exclusive interaction of Yra1 with Pcf11 and Sub2 suggests the handover of THO from the 3′-end processing complex to export-competent mRNPs.Citation84 Once 3′-end processing is completed, TREX is released from the transcription site along with its associated mRNA. Unlike yeast Yra1 and Sub2, which associate with mRNPs independent of splicing, mammalian UAP56 has a role in promoting spliceosome assembly during pre-mRNA splicing (for a review, see ref. Citation85) and Aly/REF is loaded onto mature mRNAs once splicing is completed.Citation86,Citation87 Nevertheless, UAP56, in conjunction with Aly/REF, can facilitate nuclear export of both spliced and unspliced mRNAs by recruiting the mRNA export receptor, i.e., the TAP/p15 heterodimer (yeast Mex67/Mtr2).Citation86,Citation88 Alternatively, mammalian TREX can be recruited to the 5′ end of mRNAs by the CBP80 subunit of the cap binding complex and may, thus, promote the export of intronless mRNAs.Citation89 Thus, TREX may promote efficient mRNA synthesis and ensure that correctly formed mRNPs are exported.
TREX-2 is another complex implicated in linking transcription to mRNA export and essentially functions via its association with the nuclear pore.Citation90 The TREX-2 complex consists of Sac3, Thp1, Cdc31 and the Spt-Ada-Gcn5 acetyltransferase (SAGA) complex.Citation91,Citation92 Sac3 interacts with Mex67/Mtr2 and a component of the nuclear pore complex, Nup1, and facilitates docking of mRNPs to the nuclear pore.Citation93,Citation94 Moreover, the yeast SAGA complex, via its intrinsic component Sus1, localizes actively transcribing genes close to the nuclear pore and may also help mRNA export. Furthermore, the role of the SAGA complex and Thp1 in transcription elongation suggests that TREX-2, analogous to TREX, may define a pathway connecting transcription elongation with mRNA export.Citation90 The Drosophila homolog of Sus1, ENY2, is also co-transcriptionally recruited to nascent mRNAs, indicating the evolutionarily conserved role of Sus1/ENY2 in mRNA export.Citation95 However, the association of ENY2 with components of THO instead of TREX-2 suggests that Sus1/ENY2 may participate in mRNA export, in conjunction with similar but somewhat different complexes, in higher eukaryotic cells.Citation96 Nevertheless, as compared with yeast, the detailed function of TREX and related complexes in higher eukaryotes is less clear and requires much more investigation.
The EJC complex links splicing with mRNA surveillance and translation
The EJC is deposited ~20 nucleotides upstream of ligated exon junctions of spliced mRNAs,Citation97 and influences several downstream mRNA metabolic events, including mRNA export, surveillance and translation (for a review, see refs. Citation98–Citation101). A recent comprehensive EJC protein-RNA interaction analysis has indicated that the EJC occupies the majority of exon junctions of mRNAs in cells.Citation102 The EJC comprises a four-subunit core (Y14, Magoh, eIF4AIII and MLN51) that is assembled prior to exon ligation.Citation100 Assembly of the EJC is indeed splicing-dependent and requires an intron-binding protein, IBP160.Citation103 Besides, a number of peripheral components of the EJC join the core after splicing. The core remains on the mRNA along the export pathway until translation commences.Citation104 The observation that defective splicing or 3′-end processing results in accumulation of EJC factors at transcription sites suggests that formation of the EJC occurs co-transcriptionally.Citation105
The EJC interacts with mRNA export factors UAP56, Aly/REF and TAP/p15.Citation99,Citation106 However, the observation that depletion of EJC factors does not significantly impair bulk mRNA export suggests that the EJC may be dispensable for efficient mRNA export.Citation107,Citation108 On the other hand, the EJC has been implicated in NMD, which governs quality control of mRNAs that have been newly exported and bound by the nuclear cap-binding complex CBP20/80.Citation109 NMD destroys transcripts containing a premature termination codon (PTC) during the initial round of translation and thus prevents expression of truncated proteins.Citation99,Citation101 The EJC recruits the essential NMD factor Upf3 in the nucleus and subsequently Upf2 in the cytoplasm.Citation110 A translating ribosome is stalled when it encounters a PTC and subsequently recruits additional NMD factors, including Upf1, to trigger the decay of PTC-containing transcripts.Citation111,Citation112 Knockdown of individual EJC factors inhibits NMD, indicating the importance of the EJC in NMD.Citation113
The EJC participates in NMD during the pioneer round of translation.Citation100 An activated mTOR kinase, ribosomal protein S6 kinase 1 (S6K1), can be recruited by the EJC-associated RNA-binding protein SKAR to CBP20/80-bound mRNPs, suggesting that the EJC can mediate cellular signaling pathways to regulate the pioneer-round translation and hence NMD.Citation114 Perhaps EJC-recruited S6K1 may further promote subsequent rounds of translation to increase protein yield particularly under favorable cellular conditions. Indeed, a tethering experiment has already indicated that some of the EJC components, such as the core factor Y14 and peripheral factor RNPS1, can enhance productive translation by promoting mRNA polysome association.Citation115 The Drosophila EJC factors are particularly involved in localization and/or regulated translation of some maternal mRNAs in the developing oocyte, indicating an evolutionarily conserved role of the EJC in translational control.Citation116
In addition to interacting with factors that function in mRNA export or NMD, the EJC multimerizes with itself and forms large complexes with SR proteins to promote mRNA packaging and protect mRNAs from degradation; therefore, the EJC provides a function in constitutive mRNP remodeling during mRNA maturation.Citation102 Moreover, recent evidence also indicates that individual EJC factors may have specific mRNA targets or functions. For example, eIF4AIII selectively binds to a set of dendritic mRNAs and controls their expression perhaps via NMD and, thus, modulates synaptic activities.Citation117 Interestingly, eIF4AIII interacts with an eIF4G-like protein, NOM1, which mimics the interaction between the translation initiation factors eIF4A and eIF4G, and the eIF4AIII/NOM1 participates in rRNA processing.Citation118 We have recently reported that Y14 itself can inhibit the dcapping activity of Dcp2 and prevents mRNA decay.Citation119 Therefore, EJC or NMD factors may exert different activities and provide a wide range of cellular functions.
Together, nuclear RNA processing, particularly pre-mRNA splicing, imprints mRNAs in the nucleus with a specific set of RNA binding proteins, which influences the fate of mRNAs once they reach the cytoplasm.
Beyond transcription: Epigenetic control of splicing
It has long been known that chromatin structure has a tremendous impact on transcriptional regulation.Citation120 Recently, evidence of epigenetic effects on pre-mRNA splicing has begun to emerge. In fact, an early finding that the Gcn5-related histone acetyltransferase interacts with the U2 snRNP has suggested a possible role for chromatin structure in splicing ().Citation121 Nevertheless, recent analyses of data on large-scale chromatin precipitation and high-throughput sequencing have largely unveiled the close relationship between chromatin signatures and various aspects of splicing (for a review, see refs. Citation122–Citation124). In this section, we discuss emerging evidence and underlying mechanisms of the effects of epigenetic modifications on pre-mRNA splicing.
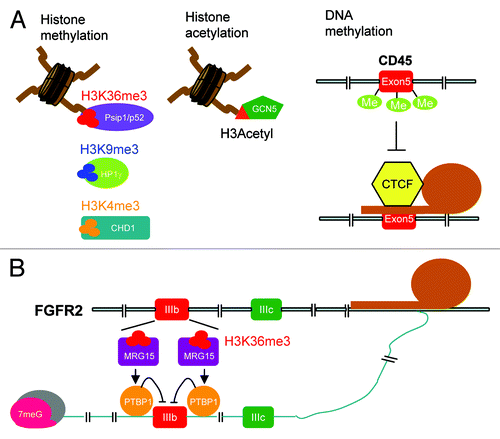
Figure 2. Chromatin modifications affect alternative splicing via adaptors. (A) Via respective adaptors, different types of histone modification (methylation and acetylation) and DNA methylation exert their effects on the regulation of alternative splicing. CpG methylation antagonizes the activity of CTCF in CD45 exon 5 inclusion. (B) H3K36me3-modified histones recruit the adaptor MRG15 to regulate PTBP1-mediated exon selection of the FGFR2 transcript.
Nucleosome positioning and exon-definition
Histones undergo extensive post-translational modifications, and different types of modifications even have combinatory effects on transcriptional regulation. Studies using chromatin precipitation coupled with deep sequencing have mapped specifically modified histones over the entire genome.Citation122,Citation124 The first identified histone signature enriched in exons is histone H3 trimethylation at lysine 36 (H3K36me3). H3K36me3 exhibits a biased distribution toward downstream exons rather than the promoter region and is less prominent in alternatively spliced exons than the constitutive ones.Citation125 The H3K36me3 signature is established by splicing-dependent recruitment of the methyltransferase HYPB/Setd2 to the elongating pol II,Citation126,Citation127 indicating that certain types of histone modification modulate pol II processivity and hence influence RNA processing. Moreover, the exon-definition model has also been suggested for the role of nucleosome positioning in pre-mRNA splicing.Citation45,Citation128 Two independent reports showed that exons, with an average size of 140~150 base-pairs and a higher GC content, are coincidently occupied by the nucleosome that wraps 147-bp long DNA.Citation129,Citation130 The transcription-independent but stably positioned nucleosomes are enriched in exons with weak splice sites but excluded from the immediate upstream/downstream intron regions and the pseudoexons flanked by functional splice sites. Such a chromatin-splice site configuration is indeed evolutionarily conserved.Citation130 Finally, an extensive mapping of 38 different histone modifications revealed a set of histone modification signatures specific to exons, which also correlates with exon selection.Citation131 These observations underscore the impact of the exon-positioned nucleosome as well as the global histone methylation signature on pre-mRNA splicing and exon utilization.
Histone methylation: Kinetic coupling vs. recruitment
It is conceivable that the nucleosome landscape affects chromatin accessibility and hence transcription processivity, which, in turn, modulates splicing.Citation123 For example, transcriptional gene silencing (TGS) can be achieved by small interfering RNA (siRNA)-mediated heterochromatin formation. Transfection of siRNAs against the downstream region of the alternatively selected extra domain I (EDI) exon of fibronectin induces locally assembled heterochromatin, which inhibits pol II elongation and hence causes EDI inclusion.Citation132 Specific modification of nucleosomes facilitates exon inclusion possibly also through a similar mechanism.Citation133 An example is given by the observation that the H3K9me3-binding transcriptional repressor HP1γ slows down the elongation rate on the variant exons of CD44 and, accordingly, causes exon inclusion (). In general, this kinetic coupling mechanism involves chromatin adaptors that read histone marks and modulate pol II elongation rate. Moreover, readers of histone modifications can interact with the splicing factors that bind to the pre-mRNA to influence splicing. This is evidenced by the mutually exclusive selection of exon IIIb or IIIc of the fibroblast growth factor receptor 2 (FGFR2) transcripts ().Citation134 Profiling of histone modifications over the FGFR2 gene revealed a tight correlation between exon inclusion and histone signatures.Citation135 When the chromatin encompassing the FGFR2 gene contains abundant H3K36me3, the exon IIIc-containing isoforms are predominantly expressed. It has been previously shown that PTBP1 suppresses exon IIIb inclusion via binding to the silencing elements around exon IIIb, which leads to a concomitant increase of the exon IIIc-containing mRNA product.Citation134 Present data indicate that the chromatin-associated protein MRG15 specifically recognizes H3K36me3 and recruits PTBP1 to prevent exon IIIb inclusion.Citation135 Moreover, it has also been reported that H3K36me3 and H3K4me3 can respectively interact with the SRSF1-interacting protein Psip1/p52 and the chromatin remodeling protein CHD1 to further recruit corresponding splicing factors to target transcripts ().Citation136,Citation137 A growing body of evidence supports the recruitment model, in which modified histones can recruit splicing factors through chromatin adaptors.
Histone acetylation
Besides histone methylation, altered acetylation status of histones and nucleosome structure can also influence splicing patterns. The histone deacetylase inhibitor, Trichostatin A (TSA), could trigger exon skipping in a minigene system.Citation138 A screen using splicing-sensitive microarrays revealed that alternative splicing of as many as 700 transcripts was perturbed upon inhibition of histone deacetylases.Citation139 Experimental evidence then indicated that inhibition of histone deacetylation prevents alternative exon inclusion of fibronectin likely by increasing RNA pol II processivity as well as reducing SRSF5/SRp40 binding to the alternatively spliced exon.Citation139 Moreover, the chromatin remodeling complex SWI/SNF modulates alternative exon selection via the activity of its catalytic subunit Brg1/Brm that reduces the rate of pol II elongation and thereby facilitates spliceosome assembly.Citation140 The physiological significance of histone acetylation on splicing has been revealed by the finding that TSA treatment mimics neuronal excitation as stimulated by KCl and induces exon 18 skipping of the neural cell adhesion molecule (NCAM) in murine neuroblastoma cells, which correlates with an increased abundance of H3K9 acetylation around exon 18.Citation141 Therefore, changes in nucleosome modification or structure can exert their effect on splicing via similar mechanisms.
DNA modification
In addition to histone modifications, DNA methylation also impacts pre-mRNA splicing. The finding that the methyl-CpG-binding protein MeCP2 can regulate pre-mRNA splicing has initially suggested the potential effect of CpG distribution and cytosine methylation on alternative splicing.Citation142 A recent bisulfite sequencing of CpG island-enriched genomic regions revealed that DNA methylation is abundant in exonic regions and correlates well with H3K36me3 levels, providing a global picture of DNA methylation and even its combinatory effect with histone methylation in exon selection.Citation143 Another study showed that the methylation-sensitive DNA binding transcriptional regulator CTCF facilitates the inclusion of CD45 exon 5 by inducing RNA pol II pausing at this exon and, interestingly, that CpG methylation antagonizes the effect of CTCF on exon 5 inclusion ().Citation144 This result provides evidence that DNA methylation influences splicing. Therefore, it is conceivable that changes in DNA methylation patterns during development or in disease, particularly in cancer, could determine alternative splicing outcome and subsequently affect transcriptome makeup.
Splicing influences chromatin modifications
Finally, the reciprocal influence of splicing on chromatin modifications has also emerged recently. A genome-wide analysis revealed that the exon-dominant positioning of H3K36me3 is enriched in intron-containing genes relative to intronless genes.Citation145 Further analysis showed that splicing inhibition impairs the recruitment of the H3K36 methyltransferase HYPB/Setd2 and then reduces the level of H3K36me3 as well as repositions H3K36me3 over the gene.Citation127,Citation145 Another evidence indicates the reaching-back mechanism mediated by the splicing regulator Hu protein. In neuronal cells, Hu targets the pre-mRNA along with the elongating pol II and subsequently induces local histone acetylation surrounding the regulated exons via inhibiting the histone deacetylase, HDAC2. This may result in increased elongation rate of subsequent rounds of transcription, which, thus, promotes exon skipping.Citation146 A very recent report reveals the influence of the first exon length in promoter-proximal histone modifications and transcription output.Citation147 Short first exons appear to increase the levels of H3K4me3 and acetylated H3K9 at promoters and, accordingly, increase the accuracy and efficiency of transcription initiation. More importantly, intron deletion reduces H3K4me3 levels at promoters, indicating splicing-dependent chromatin modification.
It has been evident that histone modifications provide another layer of alternative splicing modulation in a tissue/development-specific manner. Evidence also begins to emerge for the influence of splicing on histone modification profiles along the gene. Perhaps splicing can feedback control transcription through histone modifications.
Conclusion
We have reviewed studies of the past decade that unveil the molecular mechanisms of nuclear pre-mRNA processing and their functional interconnection with transcription. We have also discussed new results obtained by using global sequencing approaches that provide a genome-wide view of the function of individual trans-acting RNA processing factors. Recently, advanced crosslinking-immunoprecipitation and sequencing techniques enable fine-mapping of in vivo interactions between trans-factors and mRNAs even at single-nucleotide resolution.Citation148,Citation149 We may thus gain insights into how trans-factors and mRNAs constitute a highly dynamic ribonucleoproteins, and how RNA regulons are possibly assembled during mRNA processing and act in response to cellular signaling. Moreover, recent studies uncover a previously unrecognized role for chromatin modifications on pre-mRNA processing, particularly splicing regulation. We must further explore how epigenetic signals may coordinately control both transcription and alternative splicing through respective regulatory factors, and how the nucleosome modifying factors act hierarchically to optimize gene expression outcome. A further question is how environmental or heritable cues control alternative splicing by changing chromatin signatures. Finally, it is of fundamental importance to understand how the fidelity of each RNA biogenesis step is maintained and whether checkpoint mechanisms exist to control the progression from transcription to subsequent steps of RNA processing. The advent of techniques and bioinformatics would certainly unveil more comprehensive regulatory mechanisms of post-transcriptional regulation and elucidate the regulation of RNA metabolism in the context of normal physiology and disease.
References
- Moore MJ, Proudfoot NJ. Pre-mRNA processing reaches back to transcription and ahead to translation. Cell 2009; 136:688 - 700; http://dx.doi.org/10.1016/j.cell.2009.02.001; PMID: 19239889
- Cramer P, Armache KJ, Baumli S, Benkert S, Brueckner F, Buchen C, et al. Structure of eukaryotic RNA polymerases. Annu Rev Biophys 2008; 37:337 - 52; http://dx.doi.org/10.1146/annurev.biophys.37.032807.130008; PMID: 18573085
- Hsin JP, Manley JL. The RNA polymerase II CTD coordinates transcription and RNA processing. Genes Dev 2012; 26:2119 - 37; http://dx.doi.org/10.1101/gad.200303.112; PMID: 23028141
- Akhtar MS, Heidemann M, Tietjen JR, Zhang DW, Chapman RD, Eick D, et al. TFIIH kinase places bivalent marks on the carboxy-terminal domain of RNA polymerase II. Mol Cell 2009; 34:387 - 93; http://dx.doi.org/10.1016/j.molcel.2009.04.016; PMID: 19450536
- Glover-Cutter K, Larochelle S, Erickson B, Zhang C, Shokat K, Fisher RP, et al. TFIIH-associated Cdk7 kinase functions in phosphorylation of C-terminal domain Ser7 residues, promoter-proximal pausing, and termination by RNA polymerase II. Mol Cell Biol 2009; 29:5455 - 64; http://dx.doi.org/10.1128/MCB.00637-09; PMID: 19667075
- Brès V, Yoh SM, Jones KA. The multi-tasking P-TEFb complex. Curr Opin Cell Biol 2008; 20:334 - 40; http://dx.doi.org/10.1016/j.ceb.2008.04.008; PMID: 18513937
- Buratowski S. Progression through the RNA polymerase II CTD cycle. Mol Cell 2009; 36:541 - 6; http://dx.doi.org/10.1016/j.molcel.2009.10.019; PMID: 19941815
- Bonnet F, Vigneron M, Bensaude O, Dubois MF. Transcription-independent phosphorylation of the RNA polymerase II C-terminal domain (CTD) involves ERK kinases (MEK1/2). Nucleic Acids Res 1999; 27:4399 - 404; http://dx.doi.org/10.1093/nar/27.22.4399; PMID: 10536148
- Shuman S. Capping enzyme in eukaryotic mRNA synthesis. Prog Nucleic Acid Res Mol Biol 1995; 50:101 - 29; http://dx.doi.org/10.1016/S0079-6603(08)60812-0; PMID: 7754031
- Zhou Q, Li T, Price DH. RNA polymerase II elongation control. Annu Rev Biochem 2012; 81:119 - 43; http://dx.doi.org/10.1146/annurev-biochem-052610-095910; PMID: 22404626
- Ho CK, Shuman S. Distinct roles for CTD Ser-2 and Ser-5 phosphorylation in the recruitment and allosteric activation of mammalian mRNA capping enzyme. Mol Cell 1999; 3:405 - 11; http://dx.doi.org/10.1016/S1097-2765(00)80468-2; PMID: 10198643
- Schroeder SC, Schwer B, Shuman S, Bentley D. Dynamic association of capping enzymes with transcribing RNA polymerase II. Genes Dev 2000; 14:2435 - 40; http://dx.doi.org/10.1101/gad.836300; PMID: 11018011
- Ghosh A, Shuman S, Lima CD. Structural insights to how mammalian capping enzyme reads the CTD code. Mol Cell 2011; 43:299 - 310; http://dx.doi.org/10.1016/j.molcel.2011.06.001; PMID: 21683636
- Wen Y, Shatkin AJ. Transcription elongation factor hSPT5 stimulates mRNA capping. Genes Dev 1999; 13:1774 - 9; http://dx.doi.org/10.1101/gad.13.14.1774; PMID: 10421630
- Mandal SS, Chu C, Wada T, Handa H, Shatkin AJ, Reinberg D. Functional interactions of RNA-capping enzyme with factors that positively and negatively regulate promoter escape by RNA polymerase II. Proc Natl Acad Sci USA 2004; 101:7572 - 7; http://dx.doi.org/10.1073/pnas.0401493101; PMID: 15136722
- Fujinaga K, Irwin D, Huang Y, Taube R, Kurosu T, Peterlin BM. Dynamics of human immunodeficiency virus transcription: P-TEFb phosphorylates RD and dissociates negative effectors from the transactivation response element. Mol Cell Biol 2004; 24:787 - 95; http://dx.doi.org/10.1128/MCB.24.2.787-795.2004; PMID: 14701750
- Yamada T, Yamaguchi Y, Inukai N, Okamoto S, Mura T, Handa H. P-TEFb-mediated phosphorylation of hSpt5 C-terminal repeats is critical for processive transcription elongation. Mol Cell 2006; 21:227 - 37; http://dx.doi.org/10.1016/j.molcel.2005.11.024; PMID: 16427012
- St Amour CV, Sansó M, Bösken CA, Lee KM, Larochelle S, Zhang C, et al. Separate domains of fission yeast Cdk9 (P-TEFb) are required for capping enzyme recruitment and primed (Ser7-phosphorylated) Rpb1 carboxyl-terminal domain substrate recognition. Mol Cell Biol 2012; 32:2372 - 83; http://dx.doi.org/10.1128/MCB.06657-11; PMID: 22508988
- Millevoi S, Vagner S. Molecular mechanisms of eukaryotic pre-mRNA 3′ end processing regulation. Nucleic Acids Res 2010; 38:2757 - 74; http://dx.doi.org/10.1093/nar/gkp1176; PMID: 20044349
- Proudfoot NJ. Ending the message: poly(A) signals then and now. Genes Dev 2011; 25:1770 - 82; http://dx.doi.org/10.1101/gad.17268411; PMID: 21896654
- Mandel CR, Kaneko S, Zhang H, Gebauer D, Vethantham V, Manley JL, et al. Polyadenylation factor CPSF-73 is the pre-mRNA 3′-end-processing endonuclease. Nature 2006; 444:953 - 6; http://dx.doi.org/10.1038/nature05363; PMID: 17128255
- Dantonel JC, Murthy KG, Manley JL, Tora L. Transcription factor TFIID recruits factor CPSF for formation of 3′ end of mRNA. Nature 1997; 389:399 - 402; http://dx.doi.org/10.1038/38763; PMID: 9311784
- McCracken S, Fong N, Yankulov K, Ballantyne S, Pan G, Greenblatt J, et al. The C-terminal domain of RNA polymerase II couples mRNA processing to transcription. Nature 1997; 385:357 - 61; http://dx.doi.org/10.1038/385357a0; PMID: 9002523
- Komarnitsky P, Cho EJ, Buratowski S. Different phosphorylated forms of RNA polymerase II and associated mRNA processing factors during transcription. Genes Dev 2000; 14:2452 - 60; http://dx.doi.org/10.1101/gad.824700; PMID: 11018013
- Ahn SH, Kim M, Buratowski S. Phosphorylation of serine 2 within the RNA polymerase II C-terminal domain couples transcription and 3′ end processing. Mol Cell 2004; 13:67 - 76; http://dx.doi.org/10.1016/S1097-2765(03)00492-1; PMID: 14731395
- Hsin JP, Sheth A, Manley JL. RNAP II CTD phosphorylated on threonine-4 is required for histone mRNA 3′ end processing. Science 2011; 334:683 - 6; http://dx.doi.org/10.1126/science.1206034; PMID: 22053051
- Licatalosi DD, Geiger G, Minet M, Schroeder S, Cilli K, McNeil JB, et al. Functional interaction of yeast pre-mRNA 3′ end processing factors with RNA polymerase II. Mol Cell 2002; 9:1101 - 11; http://dx.doi.org/10.1016/S1097-2765(02)00518-X; PMID: 12049745
- Mayer A, Heidemann M, Lidschreiber M, Schreieck A, Sun M, Hintermair C, et al. CTD tyrosine phosphorylation impairs termination factor recruitment to RNA polymerase II. Science 2012; 336:1723 - 5; http://dx.doi.org/10.1126/science.1219651; PMID: 22745433
- Kim M, Krogan NJ, Vasiljeva L, Rando OJ, Nedea E, Greenblatt JF, et al. The yeast Rat1 exonuclease promotes transcription termination by RNA polymerase II. Nature 2004; 432:517 - 22; http://dx.doi.org/10.1038/nature03041; PMID: 15565157
- Luo W, Johnson AW, Bentley DL. The role of Rat1 in coupling mRNA 3′-end processing to transcription termination: implications for a unified allosteric-torpedo model. Genes Dev 2006; 20:954 - 65; http://dx.doi.org/10.1101/gad.1409106; PMID: 16598041
- Kim M, Vasiljeva L, Rando OJ, Zhelkovsky A, Moore C, Buratowski S. Distinct pathways for snoRNA and mRNA termination. Mol Cell 2006; 24:723 - 34; http://dx.doi.org/10.1016/j.molcel.2006.11.011; PMID: 17157255
- Kubicek K, Cerna H, Holub P, Pasulka J, Hrossova D, Loehr F, et al. Serine phosphorylation and proline isomerization in RNAP II CTD control recruitment of Nrd1. Genes Dev 2012; 26:1891 - 6; http://dx.doi.org/10.1101/gad.192781.112; PMID: 22892239
- Vasiljeva L, Kim M, Mutschler H, Buratowski S, Meinhart A. The Nrd1-Nab3-Sen1 termination complex interacts with the Ser5-phosphorylated RNA polymerase II C-terminal domain. Nat Struct Mol Biol 2008; 15:795 - 804; http://dx.doi.org/10.1038/nsmb.1468; PMID: 18660819
- Chinchilla K, Rodriguez-Molina JB, Ursic D, Finkel JS, Ansari AZ, Culbertson MR. Interactions of Sen1, Nrd1, and Nab3 with multiple phosphorylated forms of the Rpb1 C-terminal domain in Saccharomyces cerevisiae. Eukaryot Cell 2012; 11:417 - 29; http://dx.doi.org/10.1128/EC.05320-11; PMID: 22286094
- Wahl MC, Will CL, Lührmann R. The spliceosome: design principles of a dynamic RNP machine. Cell 2009; 136:701 - 18; http://dx.doi.org/10.1016/j.cell.2009.02.009; PMID: 19239890
- Neugebauer KM, Stolk JA, Roth MB. A conserved epitope on a subset of SR proteins defines a larger family of Pre-mRNA splicing factors. J Cell Biol 1995; 129:899 - 908; http://dx.doi.org/10.1083/jcb.129.4.899; PMID: 7538140
- Yuryev A, Patturajan M, Litingtung Y, Joshi RV, Gentile C, Gebara M, et al. The C-terminal domain of the largest subunit of RNA polymerase II interacts with a novel set of serine/arginine-rich proteins. Proc Natl Acad Sci USA 1996; 93:6975 - 80; http://dx.doi.org/10.1073/pnas.93.14.6975; PMID: 8692929
- Lamond AI, Spector DL. Nuclear speckles: a model for nuclear organelles. Nat Rev Mol Cell Biol 2003; 4:605 - 12; http://dx.doi.org/10.1038/nrm1172; PMID: 12923522
- Misteli T, Cáceres JF, Spector DL. The dynamics of a pre-mRNA splicing factor in living cells. Nature 1997; 387:523 - 7; http://dx.doi.org/10.1038/387523a0; PMID: 9168118
- Das R, Yu J, Zhang Z, Gygi MP, Krainer AR, Gygi SP, et al. SR proteins function in coupling RNAP II transcription to pre-mRNA splicing. Mol Cell 2007; 26:867 - 81; http://dx.doi.org/10.1016/j.molcel.2007.05.036; PMID: 17588520
- David CJ, Boyne AR, Millhouse SR, Manley JL. The RNA polymerase II C-terminal domain promotes splicing activation through recruitment of a U2AF65-Prp19 complex. Genes Dev 2011; 25:972 - 83; http://dx.doi.org/10.1101/gad.2038011; PMID: 21536736
- Muñoz MJ, de la Mata M, Kornblihtt AR. The carboxy terminal domain of RNA polymerase II and alternative splicing. Trends Biochem Sci 2010; 35:497 - 504; http://dx.doi.org/10.1016/j.tibs.2010.03.010; PMID: 20418102
- de la Mata M, Alonso CR, Kadener S, Fededa JP, Blaustein M, Pelisch F, et al. A slow RNA polymerase II affects alternative splicing in vivo. Mol Cell 2003; 12:525 - 32; http://dx.doi.org/10.1016/j.molcel.2003.08.001; PMID: 14536091
- Kornblihtt AR, de la Mata M, Fededa JP, Munoz MJ, Nogues G. Multiple links between transcription and splicing. RNA 2004; 10:1489 - 98; http://dx.doi.org/10.1261/rna.7100104; PMID: 15383674
- Gómez Acuña LI, Fiszbein A, Alló M, Schor IE, Kornblihtt AR. Connections between chromatin signatures and splicing. Wiley Interdiscip Rev RNA 2013; 4:77 - 91; http://dx.doi.org/10.1002/wrna.1142; PMID: 23074139
- Ip JY, Schmidt D, Pan Q, Ramani AK, Fraser AG, Odom DT, et al. Global impact of RNA polymerase II elongation inhibition on alternative splicing regulation. Genome Res 2011; 21:390 - 401; http://dx.doi.org/10.1101/gr.111070.110; PMID: 21163941
- Muñoz MJ, Pérez Santangelo MS, Paronetto MP, de la Mata M, Pelisch F, Boireau S, et al. DNA damage regulates alternative splicing through inhibition of RNA polymerase II elongation. Cell 2009; 137:708 - 20; http://dx.doi.org/10.1016/j.cell.2009.03.010; PMID: 19450518
- Montes M, Cloutier A, Sánchez-Hernández N, Michelle L, Lemieux B, Blanchette M, et al. TCERG1 regulates alternative splicing of the Bcl-x gene by modulating the rate of RNA polymerase II transcription. Mol Cell Biol 2012; 32:751 - 62; http://dx.doi.org/10.1128/MCB.06255-11; PMID: 22158966
- Close P, East P, Dirac-Svejstrup AB, Hartmann H, Heron M, Maslen S, et al. DBIRD complex integrates alternative mRNA splicing with RNA polymerase II transcript elongation. Nature 2012; 484:386 - 9; http://dx.doi.org/10.1038/nature10925; PMID: 22446626
- Lin S, Coutinho-Mansfield G, Wang D, Pandit S, Fu XD. The splicing factor SC35 has an active role in transcriptional elongation. Nat Struct Mol Biol 2008; 15:819 - 26; http://dx.doi.org/10.1038/nsmb.1461; PMID: 18641664
- Barboric M, Lenasi T, Chen H, Johansen EB, Guo S, Peterlin BM. 7SK snRNP/P-TEFb couples transcription elongation with alternative splicing and is essential for vertebrate development. Proc Natl Acad Sci USA 2009; 106:7798 - 803; http://dx.doi.org/10.1073/pnas.0903188106; PMID: 19416841
- Emili A, Shales M, McCracken S, Xie W, Tucker PW, Kobayashi R, et al. Splicing and transcription-associated proteins PSF and p54nrb/nonO bind to the RNA polymerase II CTD. RNA 2002; 8:1102 - 11; http://dx.doi.org/10.1017/S1355838202025037; PMID: 12358429
- Hata K, Nishimura R, Muramatsu S, Matsuda A, Matsubara T, Amano K, et al. Paraspeckle protein p54nrb links Sox9-mediated transcription with RNA processing during chondrogenesis in mice. J Clin Invest 2008; 118:3098 - 108; http://dx.doi.org/10.1172/JCI31373; PMID: 18677406
- Long JC, Caceres JF. The SR protein family of splicing factors: master regulators of gene expression. Biochem J 2009; 417:15 - 27; http://dx.doi.org/10.1042/BJ20081501; PMID: 19061484
- Irimia M, Blencowe BJ. Alternative splicing: decoding an expansive regulatory layer. Curr Opin Cell Biol 2012; 24:323 - 32; http://dx.doi.org/10.1016/j.ceb.2012.03.005; PMID: 22465326
- Xiao SH, Manley JL. Phosphorylation-dephosphorylation differentially affects activities of splicing factor ASF/SF2. EMBO J 1998; 17:6359 - 67; http://dx.doi.org/10.1093/emboj/17.21.6359; PMID: 9799243
- Murray MV, Kobayashi R, Krainer AR. The type 2C Ser/Thr phosphatase PP2Cgamma is a pre-mRNA splicing factor. Genes Dev 1999; 13:87 - 97; http://dx.doi.org/10.1101/gad.13.1.87; PMID: 9887102
- Shi Y, Reddy B, Manley JL. PP1/PP2A phosphatases are required for the second step of Pre-mRNA splicing and target specific snRNP proteins. Mol Cell 2006; 23:819 - 29; http://dx.doi.org/10.1016/j.molcel.2006.07.022; PMID: 16973434
- Huang Y, Steitz JA. SRprises along a messenger’s journey. Mol Cell 2005; 17:613 - 5; http://dx.doi.org/10.1016/j.molcel.2005.02.020; PMID: 15749011
- Twyffels L, Gueydan C, Kruys V. Shuttling SR proteins: more than splicing factors. FEBS J 2011; 278:3246 - 55; http://dx.doi.org/10.1111/j.1742-4658.2011.08274.x; PMID: 21794093
- Shen EC, Henry MF, Weiss VH, Valentini SR, Silver PA, Lee MS. Arginine methylation facilitates the nuclear export of hnRNP proteins. Genes Dev 1998; 12:679 - 91; http://dx.doi.org/10.1101/gad.12.5.679; PMID: 9499403
- Gilbert W, Guthrie C. The Glc7p nuclear phosphatase promotes mRNA export by facilitating association of Mex67p with mRNA. Mol Cell 2004; 13:201 - 12; http://dx.doi.org/10.1016/S1097-2765(04)00030-9; PMID: 14759366
- Huang Y, Yario TA, Steitz JA. A molecular link between SR protein dephosphorylation and mRNA export. Proc Natl Acad Sci USA 2004; 101:9666 - 70; http://dx.doi.org/10.1073/pnas.0403533101; PMID: 15210956
- Lai MC, Tarn WY. Hypophosphorylated ASF/SF2 binds TAP and is present in messenger ribonucleoproteins. J Biol Chem 2004; 279:31745 - 9; http://dx.doi.org/10.1074/jbc.C400173200; PMID: 15184380
- Sanford JR, Gray NK, Beckmann K, Cáceres JF. A novel role for shuttling SR proteins in mRNA translation. Genes Dev 2004; 18:755 - 68; http://dx.doi.org/10.1101/gad.286404; PMID: 15082528
- Millhouse S, Manley JL. The C-terminal domain of RNA polymerase II functions as a phosphorylation-dependent splicing activator in a heterologous protein. Mol Cell Biol 2005; 25:533 - 44; http://dx.doi.org/10.1128/MCB.25.2.533-544.2005; PMID: 15632056
- Shen H, Green MR. A pathway of sequential arginine-serine-rich domain-splicing signal interactions during mammalian spliceosome assembly. Mol Cell 2004; 16:363 - 73; http://dx.doi.org/10.1016/j.molcel.2004.10.021; PMID: 15525510
- Shen H, Green MR. RS domains contact splicing signals and promote splicing by a common mechanism in yeast through humans. Genes Dev 2006; 20:1755 - 65; http://dx.doi.org/10.1101/gad.1422106; PMID: 16766678
- Bedford MT, Leder P. The FF domain: a novel motif that often accompanies WW domains. Trends Biochem Sci 1999; 24:264 - 5; http://dx.doi.org/10.1016/S0968-0004(99)01417-6; PMID: 10390614
- Sudol M, Sliwa K, Russo T. Functions of WW domains in the nucleus. FEBS Lett 2001; 490:190 - 5; http://dx.doi.org/10.1016/S0014-5793(01)02122-6; PMID: 11223034
- Morris DP, Greenleaf AL. The splicing factor, Prp40, binds the phosphorylated carboxyl-terminal domain of RNA polymerase II. J Biol Chem 2000; 275:39935 - 43; http://dx.doi.org/10.1074/jbc.M004118200; PMID: 10978320
- Kao HY, Siliciano PG. Identification of Prp40, a novel essential yeast splicing factor associated with the U1 small nuclear ribonucleoprotein particle. Mol Cell Biol 1996; 16:960 - 7; PMID: 8622699
- Abovich N, Rosbash M. Cross-intron bridging interactions in the yeast commitment complex are conserved in mammals. Cell 1997; 89:403 - 12; http://dx.doi.org/10.1016/S0092-8674(00)80221-4; PMID: 9150140
- Allen M, Friedler A, Schon O, Bycroft M. The structure of an FF domain from human HYPA/FBP11. J Mol Biol 2002; 323:411 - 6; http://dx.doi.org/10.1016/S0022-2836(02)00968-3; PMID: 12381297
- Bedford MT, Chan DC, Leder P. FBP WW domains and the Abl SH3 domain bind to a specific class of proline-rich ligands. EMBO J 1997; 16:2376 - 83; http://dx.doi.org/10.1093/emboj/16.9.2376; PMID: 9171351
- Bedford MT, Reed R, Leder P. WW domain-mediated interactions reveal a spliceosome-associated protein that binds a third class of proline-rich motif: the proline glycine and methionine-rich motif. Proc Natl Acad Sci USA 1998; 95:10602 - 7; http://dx.doi.org/10.1073/pnas.95.18.10602; PMID: 9724750
- Lin KT, Lu RM, Tarn WY. The WW domain-containing proteins interact with the early spliceosome and participate in pre-mRNA splicing in vivo. Mol Cell Biol 2004; 24:9176 - 85; http://dx.doi.org/10.1128/MCB.24.20.9176-9185.2004; PMID: 15456888
- Goldstrohm AC, Albrecht TR, Suñé C, Bedford MT, Garcia-Blanco MA. The transcription elongation factor CA150 interacts with RNA polymerase II and the pre-mRNA splicing factor SF1. Mol Cell Biol 2001; 21:7617 - 28; http://dx.doi.org/10.1128/MCB.21.22.7617-7628.2001; PMID: 11604498
- Katahira J. mRNA export and the TREX complex. Biochim Biophys Acta 2012; 1819:507 - 13; http://dx.doi.org/10.1016/j.bbagrm.2011.12.001; PMID: 22178508
- Chanarat S, Seizl M, Strässer K. The Prp19 complex is a novel transcription elongation factor required for TREX occupancy at transcribed genes. Genes Dev 2011; 25:1147 - 58; http://dx.doi.org/10.1101/gad.623411; PMID: 21576257
- Strässer K, Masuda S, Mason P, Pfannstiel J, Oppizzi M, Rodriguez-Navarro S, et al. TREX is a conserved complex coupling transcription with messenger RNA export. Nature 2002; 417:304 - 8; http://dx.doi.org/10.1038/nature746; PMID: 11979277
- Abruzzi KC, Lacadie S, Rosbash M. Biochemical analysis of TREX complex recruitment to intronless and intron-containing yeast genes. EMBO J 2004; 23:2620 - 31; http://dx.doi.org/10.1038/sj.emboj.7600261; PMID: 15192704
- Kim M, Ahn SH, Krogan NJ, Greenblatt JF, Buratowski S. Transitions in RNA polymerase II elongation complexes at the 3′ ends of genes. EMBO J 2004; 23:354 - 64; http://dx.doi.org/10.1038/sj.emboj.7600053; PMID: 14739930
- Johnson SA, Cubberley G, Bentley DL. Cotranscriptional recruitment of the mRNA export factor Yra1 by direct interaction with the 3′ end processing factor Pcf11. Mol Cell 2009; 33:215 - 26; http://dx.doi.org/10.1016/j.molcel.2008.12.007; PMID: 19110458
- Shen H. UAP56- a key player with surprisingly diverse roles in pre-mRNA splicing and nuclear export. BMB Rep 2009; 42:185 - 8; http://dx.doi.org/10.5483/BMBRep.2009.42.4.185; PMID: 19403039
- Zhou Z, Luo MJ, Straesser K, Katahira J, Hurt E, Reed R. The protein Aly links pre-messenger-RNA splicing to nuclear export in metazoans. Nature 2000; 407:401 - 5; http://dx.doi.org/10.1038/35030160; PMID: 11014198
- Shen H, Zheng X, Shen J, Zhang L, Zhao R, Green MR. Distinct activities of the DExD/H-box splicing factor hUAP56 facilitate stepwise assembly of the spliceosome. Genes Dev 2008; 22:1796 - 803; http://dx.doi.org/10.1101/gad.1657308; PMID: 18593880
- Strässer K, Hurt E. Yra1p, a conserved nuclear RNA-binding protein, interacts directly with Mex67p and is required for mRNA export. EMBO J 2000; 19:410 - 20; http://dx.doi.org/10.1093/emboj/19.3.410; PMID: 10722314
- Cheng H, Dufu K, Lee CS, Hsu JL, Dias A, Reed R. Human mRNA export machinery recruited to the 5′ end of mRNA. Cell 2006; 127:1389 - 400; http://dx.doi.org/10.1016/j.cell.2006.10.044; PMID: 17190602
- García-Oliver E, García-Molinero V, Rodríguez-Navarro S. mRNA export and gene expression: The SAGA-TREX-2 connection. Biochim Biophys Acta 2012; 1819:555 - 65; http://dx.doi.org/10.1016/j.bbagrm.2011.11.011; PMID: 22178374
- Rodríguez-Navarro S, Fischer T, Luo MJ, Antúnez O, Brettschneider S, Lechner J, et al. Sus1, a functional component of the SAGA histone acetylase complex and the nuclear pore-associated mRNA export machinery. Cell 2004; 116:75 - 86; http://dx.doi.org/10.1016/S0092-8674(03)01025-0; PMID: 14718168
- Köhler A, Hurt E. Exporting RNA from the nucleus to the cytoplasm. Nat Rev Mol Cell Biol 2007; 8:761 - 73; http://dx.doi.org/10.1038/nrm2255; PMID: 17786152
- Lei EP, Stern CA, Fahrenkrog B, Krebber H, Moy TI, Aebi U, et al. Sac3 is an mRNA export factor that localizes to cytoplasmic fibrils of nuclear pore complex. Mol Biol Cell 2003; 14:836 - 47; http://dx.doi.org/10.1091/mbc.E02-08-0520; PMID: 12631707
- Jani D, Lutz S, Marshall NJ, Fischer T, Köhler A, Ellisdon AM, et al. Sus1, Cdc31, and the Sac3 CID region form a conserved interaction platform that promotes nuclear pore association and mRNA export. Mol Cell 2009; 33:727 - 37; http://dx.doi.org/10.1016/j.molcel.2009.01.033; PMID: 19328066
- Kurshakova MM, Krasnov AN, Kopytova DV, Shidlovskii YV, Nikolenko JV, Nabirochkina EN, et al. SAGA and a novel Drosophila export complex anchor efficient transcription and mRNA export to NPC. EMBO J 2007; 26:4956 - 65; http://dx.doi.org/10.1038/sj.emboj.7601901; PMID: 18034162
- Kopytova DV, Orlova AV, Krasnov AN, Gurskiy DY, Nikolenko JV, Nabirochkina EN, et al. Multifunctional factor ENY2 is associated with the THO complex and promotes its recruitment onto nascent mRNA. Genes Dev 2010; 24:86 - 96; http://dx.doi.org/10.1101/gad.550010; PMID: 20048002
- Le Hir H, Izaurralde E, Maquat LE, Moore MJ. The spliceosome deposits multiple proteins 20-24 nucleotides upstream of mRNA exon-exon junctions. EMBO J 2000; 19:6860 - 9; http://dx.doi.org/10.1093/emboj/19.24.6860; PMID: 11118221
- Tange TO, Nott A, Moore MJ. The ever-increasing complexities of the exon junction complex. Curr Opin Cell Biol 2004; 16:279 - 84; http://dx.doi.org/10.1016/j.ceb.2004.03.012; PMID: 15145352
- Chang YF, Imam JS, Wilkinson MF. The nonsense-mediated decay RNA surveillance pathway. Annu Rev Biochem 2007; 76:51 - 74; http://dx.doi.org/10.1146/annurev.biochem.76.050106.093909; PMID: 17352659
- Bono F, Gehring NH. Assembly, disassembly and recycling: the dynamics of exon junction complexes. RNA Biol 2011; 8:24 - 9; http://dx.doi.org/10.4161/rna.8.1.13618; PMID: 21289489
- Schoenberg DR, Maquat LE. Regulation of cytoplasmic mRNA decay. Nat Rev Genet 2012; 13:246 - 59; http://dx.doi.org/10.1038/nrg3254; PMID: 22392217
- Singh G, Kucukural A, Cenik C, Leszyk JD, Shaffer SA, Weng Z, et al. The cellular EJC interactome reveals higher-order mRNP structure and an EJC-SR protein nexus. Cell 2012; 151:750 - 64; http://dx.doi.org/10.1016/j.cell.2012.10.007; PMID: 23084401
- Ideue T, Sasaki YT, Hagiwara M, Hirose T. Introns play an essential role in splicing-dependent formation of the exon junction complex. Genes Dev 2007; 21:1993 - 8; http://dx.doi.org/10.1101/gad.1557907; PMID: 17675447
- Dostie J, Dreyfuss G. Translation is required to remove Y14 from mRNAs in the cytoplasm. Curr Biol 2002; 12:1060 - 7; http://dx.doi.org/10.1016/S0960-9822(02)00902-8; PMID: 12121612
- Custódio N, Carvalho C, Condado I, Antoniou M, Blencowe BJ, Carmo-Fonseca M. In vivo recruitment of exon junction complex proteins to transcription sites in mammalian cell nuclei. RNA 2004; 10:622 - 33; http://dx.doi.org/10.1261/rna.5258504; PMID: 15037772
- Le Hir H, Gatfield D, Izaurralde E, Moore MJ. The exon-exon junction complex provides a binding platform for factors involved in mRNA export and nonsense-mediated mRNA decay. EMBO J 2001; 20:4987 - 97; http://dx.doi.org/10.1093/emboj/20.17.4987; PMID: 11532962
- Gatfield D, Izaurralde E. REF1/Aly and the additional exon junction complex proteins are dispensable for nuclear mRNA export. J Cell Biol 2002; 159:579 - 88; http://dx.doi.org/10.1083/jcb.200207128; PMID: 12438415
- Longman D, Johnstone IL, Cáceres JF. The Ref/Aly proteins are dispensable for mRNA export and development in Caenorhabditis elegans. RNA 2003; 9:881 - 91; http://dx.doi.org/10.1261/rna.5420503; PMID: 12810921
- Ishigaki Y, Li X, Serin G, Maquat LE. Evidence for a pioneer round of mRNA translation: mRNAs subject to nonsense-mediated decay in mammalian cells are bound by CBP80 and CBP20. Cell 2001; 106:607 - 17; http://dx.doi.org/10.1016/S0092-8674(01)00475-5; PMID: 11551508
- Singh G, Jakob S, Kleedehn MG, Lykke-Andersen J. Communication with the exon-junction complex and activation of nonsense-mediated decay by human Upf proteins occur in the cytoplasm. Mol Cell 2007; 27:780 - 92; http://dx.doi.org/10.1016/j.molcel.2007.06.030; PMID: 17803942
- Kashima I, Yamashita A, Izumi N, Kataoka N, Morishita R, Hoshino S, et al. Binding of a novel SMG-1-Upf1-eRF1-eRF3 complex (SURF) to the exon junction complex triggers Upf1 phosphorylation and nonsense-mediated mRNA decay. Genes Dev 2006; 20:355 - 67; http://dx.doi.org/10.1101/gad.1389006; PMID: 16452507
- Isken O, Kim YK, Hosoda N, Mayeur GL, Hershey JW, Maquat LE. Upf1 phosphorylation triggers translational repression during nonsense-mediated mRNA decay. Cell 2008; 133:314 - 27; http://dx.doi.org/10.1016/j.cell.2008.02.030; PMID: 18423202
- Gehring NH, Kunz JB, Neu-Yilik G, Breit S, Viegas MH, Hentze MW, et al. Exon-junction complex components specify distinct routes of nonsense-mediated mRNA decay with differential cofactor requirements. Mol Cell 2005; 20:65 - 75; http://dx.doi.org/10.1016/j.molcel.2005.08.012; PMID: 16209946
- Ma XM, Yoon SO, Richardson CJ, Jülich K, Blenis J. SKAR links pre-mRNA splicing to mTOR/S6K1-mediated enhanced translation efficiency of spliced mRNAs. Cell 2008; 133:303 - 13; http://dx.doi.org/10.1016/j.cell.2008.02.031; PMID: 18423201
- Nott A, Le Hir H, Moore MJ. Splicing enhances translation in mammalian cells: an additional function of the exon junction complex. Genes Dev 2004; 18:210 - 22; http://dx.doi.org/10.1101/gad.1163204; PMID: 14752011
- Lasko P. Posttranscriptional regulation in Drosophila oocytes and early embryos. Wiley Interdiscip Rev RNA 2011; 2:408 - 16; http://dx.doi.org/10.1002/wrna.70; PMID: 21957026
- Giorgi C, Yeo GW, Stone ME, Katz DB, Burge C, Turrigiano G, et al. The EJC factor eIF4AIII modulates synaptic strength and neuronal protein expression. Cell 2007; 130:179 - 91; http://dx.doi.org/10.1016/j.cell.2007.05.028; PMID: 17632064
- Alexandrov A, Colognori D, Steitz JA. Human eIF4AIII interacts with an eIF4G-like partner, NOM1, revealing an evolutionarily conserved function outside the exon junction complex. Genes Dev 2011; 25:1078 - 90; http://dx.doi.org/10.1101/gad.2045411; PMID: 21576267
- Chuang TW, Chang WL, Lee KM, Tarn WY. The RNA-binding protein Y14 inhibits mRNA decapping and modulates processing body formation. Mol Biol Cell 2013; 24:1 - 13; http://dx.doi.org/10.1091/mbc.E12-03-0217; PMID: 23115303
- Weake VM, Workman JL. Inducible gene expression: diverse regulatory mechanisms. Nat Rev Genet 2010; 11:426 - 37; http://dx.doi.org/10.1038/nrg2781; PMID: 20421872
- Martinez E, Palhan VB, Tjernberg A, Lymar ES, Gamper AM, Kundu TK, et al. Human STAGA complex is a chromatin-acetylating transcription coactivator that interacts with pre-mRNA splicing and DNA damage-binding factors in vivo. Mol Cell Biol 2001; 21:6782 - 95; http://dx.doi.org/10.1128/MCB.21.20.6782-6795.2001; PMID: 11564863
- Jiang C, Pugh BF. Nucleosome positioning and gene regulation: advances through genomics. Nat Rev Genet 2009; 10:161 - 72; http://dx.doi.org/10.1038/nrg2522; PMID: 19204718
- Luco RF, Allo M, Schor IE, Kornblihtt AR, Misteli T. Epigenetics in alternative pre-mRNA splicing. Cell 2011; 144:16 - 26; http://dx.doi.org/10.1016/j.cell.2010.11.056; PMID: 21215366
- Hurd PJ, Nelson CJ. Advantages of next-generation sequencing versus the microarray in epigenetic research. Brief Funct Genomic Proteomic 2009; 8:174 - 83; http://dx.doi.org/10.1093/bfgp/elp013; PMID: 19535508
- Kolasinska-Zwierz P, Down T, Latorre I, Liu T, Liu XS, Ahringer J. Differential chromatin marking of introns and expressed exons by H3K36me3. Nat Genet 2009; 41:376 - 81; http://dx.doi.org/10.1038/ng.322; PMID: 19182803
- Yoh SM, Lucas JS, Jones KA. The Iws1:Spt6:CTD complex controls cotranscriptional mRNA biosynthesis and HYPB/Setd2-mediated histone H3K36 methylation. Genes Dev 2008; 22:3422 - 34; http://dx.doi.org/10.1101/gad.1720008; PMID: 19141475
- de Almeida SF, Grosso AR, Koch F, Fenouil R, Carvalho S, Andrade J, et al. Splicing enhances recruitment of methyltransferase HYPB/Setd2 and methylation of histone H3 Lys36. Nat Struct Mol Biol 2011; 18:977 - 83; http://dx.doi.org/10.1038/nsmb.2123; PMID: 21792193
- Kornblihtt AR, Schor IE, Allo M, Blencowe BJ. When chromatin meets splicing. Nat Struct Mol Biol 2009; 16:902 - 3; http://dx.doi.org/10.1038/nsmb0909-902; PMID: 19739285
- Schwartz S, Meshorer E, Ast G. Chromatin organization marks exon-intron structure. Nat Struct Mol Biol 2009; 16:990 - 5; http://dx.doi.org/10.1038/nsmb.1659; PMID: 19684600
- Tilgner H, Nikolaou C, Althammer S, Sammeth M, Beato M, Valcárcel J, et al. Nucleosome positioning as a determinant of exon recognition. Nat Struct Mol Biol 2009; 16:996 - 1001; http://dx.doi.org/10.1038/nsmb.1658; PMID: 19684599
- Andersson R, Enroth S, Rada-Iglesias A, Wadelius C, Komorowski J. Nucleosomes are well positioned in exons and carry characteristic histone modifications. Genome Res 2009; 19:1732 - 41; http://dx.doi.org/10.1101/gr.092353.109; PMID: 19687145
- Alló M, Buggiano V, Fededa JP, Petrillo E, Schor I, de la Mata M, et al. Control of alternative splicing through siRNA-mediated transcriptional gene silencing. Nat Struct Mol Biol 2009; 16:717 - 24; http://dx.doi.org/10.1038/nsmb.1620; PMID: 19543290
- Saint-André V, Batsché E, Rachez C, Muchardt C. Histone H3 lysine 9 trimethylation and HP1γ favor inclusion of alternative exons. Nat Struct Mol Biol 2011; 18:337 - 44; http://dx.doi.org/10.1038/nsmb.1995; PMID: 21358630
- Carstens RP, Wagner EJ, Garcia-Blanco MA. An intronic splicing silencer causes skipping of the IIIb exon of fibroblast growth factor receptor 2 through involvement of polypyrimidine tract binding protein. Mol Cell Biol 2000; 20:7388 - 400; http://dx.doi.org/10.1128/MCB.20.19.7388-7400.2000; PMID: 10982855
- Luco RF, Pan Q, Tominaga K, Blencowe BJ, Pereira-Smith OM, Misteli T. Regulation of alternative splicing by histone modifications. Science 2010; 327:996 - 1000; http://dx.doi.org/10.1126/science.1184208; PMID: 20133523
- Sims RJ 3rd, Millhouse S, Chen CF, Lewis BA, Erdjument-Bromage H, Tempst P, et al. Recognition of trimethylated histone H3 lysine 4 facilitates the recruitment of transcription postinitiation factors and pre-mRNA splicing. Mol Cell 2007; 28:665 - 76; http://dx.doi.org/10.1016/j.molcel.2007.11.010; PMID: 18042460
- Pradeepa MM, Sutherland HG, Ule J, Grimes GR, Bickmore WA. Psip1/Ledgf p52 binds methylated histone H3K36 and splicing factors and contributes to the regulation of alternative splicing. PLoS Genet 2012; 8:e1002717; http://dx.doi.org/10.1371/journal.pgen.1002717; PMID: 22615581
- Nogues G, Kadener S, Cramer P, Bentley D, Kornblihtt AR. Transcriptional activators differ in their abilities to control alternative splicing. J Biol Chem 2002; 277:43110 - 4; http://dx.doi.org/10.1074/jbc.M208418200; PMID: 12221105
- Hnilicová J, Hozeifi S, Dušková E, Icha J, Tománková T, Staněk D. Histone deacetylase activity modulates alternative splicing. PLoS One 2011; 6:e16727; http://dx.doi.org/10.1371/journal.pone.0016727; PMID: 21311748
- Batsché E, Yaniv M, Muchardt C. The human SWI/SNF subunit Brm is a regulator of alternative splicing. Nat Struct Mol Biol 2006; 13:22 - 9; http://dx.doi.org/10.1038/nsmb1030; PMID: 16341228
- Schor IE, Rascovan N, Pelisch F, Alló M, Kornblihtt AR. Neuronal cell depolarization induces intragenic chromatin modifications affecting NCAM alternative splicing. Proc Natl Acad Sci USA 2009; 106:4325 - 30; http://dx.doi.org/10.1073/pnas.0810666106; PMID: 19251664
- Young JI, Hong EP, Castle JC, Crespo-Barreto J, Bowman AB, Rose MF, et al. Regulation of RNA splicing by the methylation-dependent transcriptional repressor methyl-CpG binding protein 2. Proc Natl Acad Sci USA 2005; 102:17551 - 8; http://dx.doi.org/10.1073/pnas.0507856102; PMID: 16251272
- Hodges E, Smith AD, Kendall J, Xuan Z, Ravi K, Rooks M, et al. High definition profiling of mammalian DNA methylation by array capture and single molecule bisulfite sequencing. Genome Res 2009; 19:1593 - 605; http://dx.doi.org/10.1101/gr.095190.109; PMID: 19581485
- Shukla S, Kavak E, Gregory M, Imashimizu M, Shutinoski B, Kashlev M, et al. CTCF-promoted RNA polymerase II pausing links DNA methylation to splicing. Nature 2011; 479:74 - 9; http://dx.doi.org/10.1038/nature10442; PMID: 21964334
- Kim S, Kim H, Fong N, Erickson B, Bentley DL. Pre-mRNA splicing is a determinant of histone H3K36 methylation. Proc Natl Acad Sci USA 2011; 108:13564 - 9; http://dx.doi.org/10.1073/pnas.1109475108; PMID: 21807997
- Zhou HL, Hinman MN, Barron VA, Geng C, Zhou G, Luo G, et al. Hu proteins regulate alternative splicing by inducing localized histone hyperacetylation in an RNA-dependent manner. Proc Natl Acad Sci USA 2011; 108:E627 - 35; http://dx.doi.org/10.1073/pnas.1103344108; PMID: 21808035
- Bieberstein NI, Carrillo Oesterreich F, Straube K, Neugebauer KM. First exon length controls active chromatin signatures and transcription. Cell Rep 2012; 2:62 - 8; http://dx.doi.org/10.1016/j.celrep.2012.05.019; PMID: 22840397
- König J, Zarnack K, Rot G, Curk T, Kayikci M, Zupan B, et al. iCLIP reveals the function of hnRNP particles in splicing at individual nucleotide resolution. Nat Struct Mol Biol 2010; 17:909 - 15; http://dx.doi.org/10.1038/nsmb.1838; PMID: 20601959
- Hafner M, Lianoglou S, Tuschl T, Betel D. Genome-wide identification of miRNA targets by PAR-CLIP. Methods 2012; 58:94 - 105; http://dx.doi.org/10.1016/j.ymeth.2012.08.006; PMID: 22926237