Abstract
Role of miRNAs in dyspraxia, dyslexia, and specific language impairment
SUMOylation of Akt regulates alternative splicing and cell cycle
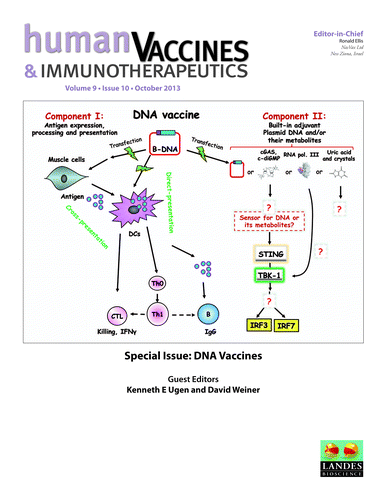
Self-adjuvanted RNActive vaccines
mRNA translation: 4E-BP restrains eIF4E phosphorylation
Role of miRNAs in Dyspraxia, Dyslexia, and Specific Language Impairment
Alexander Rudov, Marco Bruno Luigi Rocchi, Augusto Accorsi, Giorgio Spada, Antonio Domenico Procopio, Fabiola Olivieri, Maria Rita Rippo, and Maria Cristina Albertini
Disorders of human communication abilities can be classified into speech and language disorders. Speech disorders (e.g., dyspraxia) affect the sound generation and sequencing, while language disorders (e.g., dyslexia and specific language impairment, or SLI) are deficits in the encoding and decoding of language according to its rules (reading, spelling, grammar). The diagnosis of such disorders is often complicated, especially when a patient presents more than one disorder at the same time. A new review by Dr Maria Cristina Albertini and colleagues focuses on these challenges. In an attempt to identify putative miRNAs that may have a key role in dyspraxia, dyslexia, and SLI, the authors combined data available from the literature with an in silico approach. They identified new miRNAs, which could have an important impact on the three diseases. Further, they relate those miRNAs to the axon guidance pathway and discuss possible interactions and the role of likely deregulated proteins. Finally, potential differences in expressional deregulation and its role in the improvement of diagnosis are described. The data obtained are now to be validated experimentally.Citation1 . https://www.landesbioscience.com/journals/epigenetics/article/26026/
SUMOylation of Akt Regulates Alternative Splicing and Cell Cycle
Guillermo Risso, Federico Pelisch, Berta Pozzi, Pablo Mammi, Matías Blaustein, Alejandro Colman-Lerner, and Anabella Srebrow
Akt/PKB is a key signaling molecule in higher eukaryotes and a crucial protein kinase in human health and disease. Phosphorylation, acetylation, and ubiquitylation have been reported as important regulatory post-translational modifications of this kinase. A new study by Dr Anabella Srebrow and colleagues showed that Akt is modified by SUMO conjugation, with lysine residues 276 and 301 being the major SUMO attachment sites within this protein. They found that phosphorylation and SUMOylation of Akt appear as independent events. However, decreasing Akt SUMOylation levels severely affected the role of this kinase as a regulator of fibronectin and Bcl-x alternative splicing. Moreover, the authors observed that the Akt mutant (Akt E17K) found in several human tumors displays increased levels of SUMOylation and also an enhanced capacity to regulate fibronectin splicing patterns. This splicing regulatory activity was completely abolished by decreasing Akt E17K SUMO conjugation levels. Additionally, they found that SUMOylation controls Akt regulatory function at G1/S transition during cell cycle progression. These study findings reveal SUMO conjugation as a novel level of regulation for Akt activity, opening new areas of exploration related to the molecular mechanisms involved in the diverse cellular functions of this kinase.Citation2 . https://www.landesbioscience.com/journals/cc/article/26183/
Self-Adjuvanted RNActive Vaccines
Karl-Josef Kallen, Regina Heidenreich, Margit Schnee, Benjamin Petsch, Thomas Schlake, Andreas Thess, Patrick Baumhof, Birgit Scheel, Sven D Koch, and Mariola Fotin-Mleczek
Nucleotide-based vaccines represent an enticing, novel approach to vaccination. Despite intensive research in the last decades, DNA vaccines have not yet achieved the break-through in humans. In a recent review, Dr Karl-Josef Kallen and co-workers describe how a vaccine based on mRNA might represent a suitable alternative for nucleotide-based vaccination. Their novel immunization technology, called RNActive vaccines, has two important characteristics: mRNA molecules are used whose protein expression capacity has been enhanced by 4 to 5 orders of magnitude by modifications of the nucleotide sequence with the naturally occurring nucleotides A (adenosine), G (guanosine), C (cytosine), U (uridine) that do not affect the primary amino acid sequence. Second, they are complexed with protamine and thus activate the immune system by involvement of toll-like receptor (TLR) 7. Essentially, this bestows self-adjuvant activity on RNActive vaccines. The authors summarize preclinical and clinical data on RNActive vaccines against various diseases such as influenza or cancer. RNActive vaccines were shown to induce strong, balanced immune responses comprising humoral and cellular responses, effector and memory responses, as well as activation of important subpopulations of immune cells, such as Th1 and Th2 cells. Pre-germinal center and germinal center B cells were detected in human patients upon vaccination. RNActive vaccines successfully protected against lethal challenges with a variety of different influenza strains in preclinical models. Anti-tumor activity was observed preclinically under therapeutic as well as prophylactic conditions. Initial clinical experiences showed a favorable safety profile of RNActive vaccines and preclinical immunogenicity could be successfully translated to humans.Citation3 . https://www.landesbioscience.com/journals/vaccines/article/25181/
mRNA Translation: 4E-BP Restrains eIF4E Phosphorylation
David Müller, Charline Lasfargues, Sally El Khawand, Amandine Alard, Robert J. Schneider, Corinne Bousquet, Stéphane Pyronnet, and Yvan Martineau
In eukaryotes, mRNA translation is dependent on the cap-binding protein eIF4E. Through its simultaneous interaction with the mRNA cap structure and with the ribosome-associated eIF4G adaptor protein, eIF4E physically posits the ribosome at the 5′ extremity of capped mRNA. eIF4E activity is regulated by phosphorylation on a unique site by the eIF4G-associated kinase MNK. eIF4E assembly with the eIF4G-MNK sub-complex can be however antagonized by the hypophosphorylated forms of eIF4E-binding protein (4E-BP). In a recent study, Drs Pyronnet and Martineau showed that eIF4E phosphorylation is dramatically affected by disruption of eIF4E-eIF4G interaction, independently of changes in MNK expression. eIF4E phosphorylation was actually strongly downregulated upon eIF4G shutdown or upon sequestration by hypophosphorylated 4E-BP, consequent to mTOR inhibition. Downregulation of 4E-BP rendered eIF4E phosphorylation insensitive to mTOR inhibition. These data highlight the important role of 4E-BP in regulating eIF4E phosphorylation independently of changes in MNK expression.Citation4 . https://www.landesbioscience.com/journals/translation/article/25819/
References
- Rudov A, Rocchi MBL, Accorsi A, Spada G, Procopio AD, Olivieri F, Rippo MR, Albertini MC. Putative miRNAs for the diagnosis of dyslexia, dyspraxia, and specific language impairment. Epigenetics 2013; 8; In press http://dx.doi.org/10.4161/epi.26026; PMID: 23949389
- Risso G, Pelisch F, Pozzi B, Mammi P, Blaustein M, Colman-Lerner A, Srebrow A. Modification of Akt by SUMO conjugation regulates alternative splicing and cell cycle. Cell Cycle 2013; 12:3165 - 74; http://dx.doi.org/10.4161/cc.26183; PMID: 24013425
- Kallen K-J, Heidenreich R, Schnee M, Petsch B, Schlake T, Thess A, Baumhof P, Scheel B, Koch SD, Fotin-Mleczek M. A novel, disruptive vaccination technology: Self-adjuvanted RNActive (®) vaccines. Hum Vaccin Immunother 2013; 9; In press http://dx.doi.org/10.4161/hv.25181; PMID: 23921513
- Müller D, Lasfargues C, El Khawand S, Alard A, Schneider RJ, Bousquet C, Pyronnet S, Martineau Y. 4E-BP restrains eIF4E phosphorylation. Translation 2013; 1 In press