Abstract
Heat stress compromises intestinal integrity which may partially explain its negative effects on animal health and productivity. Research suggests that challenged intestinal barrier function improves with dietary dairy products in various models. Thus, the study objective was to evaluate the effects of bovine milk whey protein (WP) and colostral whey protein (CWP) on intestinal integrity in heat-stressed pigs. Crossbred gilts (39 ± 3 kg body weight) were fed 1 of 4 diets (n = 8 pigs/diet): control (Ct), control diet containing an 80% WP and 20% CWP product (WP80), control diet containing a 98% WP and 2% CWP product (WP98), and control diet containing a 100% WP product (WP100). After 7d on experimental diets, pigs were exposed to constant heat stress conditions (32 °C) for 24h. There were no treatment differences in growth or body temperature indices prior to heat stress. During heat exposure, both rectal temperature and respiration rate increased (+0.85 °C and 3-fold, respectively; P < 0.01), and feed intake and body weight decreased (44% and -0.5kg, respectively; P < 0.01), but neither variable was affected by dietary treatments. Plasma L-lactate and D-lactate concentrations increased (36%; P < 0.01) and tended to increase (19%; P = 0.09) with heat stress. After 24h of heat exposure, WP100-fed pigs had lower plasma D-lactate relative to Ct-fed pigs. Ileal transepithelial electrical resistance was decreased (37%; P = 0.02) in WP80 pigs, compared with controls. No differences were detected in other intestinal integrity ex vivo measurements. These data demonstrate that dietary WP and CWP did not mitigate intestinal integrity dysfunction during severe heat stress.
Introduction
Heat stress negatively affects animal agriculture by reducing productivity and jeopardizing animal welfare. For the global swine industry, the effects of HS on growth, carcass quality, health and reproduction undermine efforts to improve efficiency and sustainability.Citation1 Further, the deleterious effects of HS will be aggravated if climate continues to warm as predicted.Citation2 In addition, genetic selection for rapid skeletal muscle growth might increase pigs’ susceptibility to HS, as enhanced lean tissue accretion is accompanied by increased metabolic heat production.Citation3 Therefore, developing nutritional strategies to alleviate the effects of HS would be a key tool to maximize efficient animal protein production during the warm summer months.Citation4
Heat stress compromises intestinal barrier function in a variety of species,Citation5 and this might partially explain its effect on animal production. In agreement, we have repeatedly demonstrated that heat-stressed growing pigs have reduced intestinal integrity and function.Citation6-Citation8 This is caused by the re-distribution of blood flow to the periphery for increased heat dissipation, which reduces oxygen and nutrient supply to intestinal tissues, resulting in enterocyte damage and increased permeability to luminal content and pathogens.Citation9 The increased passage of bacterial components (e.g., LPS) and bacteria into portal and ultimately systemic circulation are partially responsible for the pathophysiology of heat related illnesses, as reducing intestinal bacterial loadCitation10 or neutralizing plasma LPSCitation11 increase heat stroke survival. Hence, approaches attempting to preserve and restore intestinal barrier function might improve animal production and wellbeing during environmentally-induced hyperthermia.
Dietary dairy products have been demonstrated to improve gut health. For instance, milk, colostrum, and whey protein supplementation are beneficial in models of induced intestinal damage in both mice and humans,Citation12,Citation13 as an ulcerative colitis treatment,Citation14 and in vitro in response to a tight junction disruptor.Citation15 Interestingly, dietary dairy products ameliorated the effects of HS on intestinal barrier function in both miceCitation15 and a human colonic cell line.Citation16
Thus, current study objectives were to determine the effects of dietary bovine WP concentrate and CWP concentrate on intestinal integrity parameters and blood biomarkers of “leaky gut” in heat-stressed pigs. We hypothesized that feeding dairy products would prevent or at least ameliorate the deleterious effects of HS on gut permeability.
Materials and Methods
Animals and Experimental Design
Colostrum whey protein concentrate was obtained from Sterling Technologies and was blended with conventional WP concentrate (Main Street Ingredients). Iowa State University Institutional Animal Care and Use Committee approved all procedures involving animals. Thirty two crossbred gilts (39 ± 3 kg body weight) were stratified by body weight and then randomly assigned to 1 of 4 diets: 1) control (Ct), 2) the control diet containing an 80% WP and 20% CWP test product (WP80); 3) the control diet containing a 98% WP and 2% CWP test product (WP98); and 4) the control diet containing a 100% WP test product (WP100). Test dairy products were similar in nutrient composition () and constituted 7% of the respective diets in order to provide 100 g/d of protein. Other than the added test dairy products, all diets were similar in ingredient and nutrient composition and were formulated to meet or exceed the estimated requirementsCitation17 for essential amino acids, protein, minerals, and vitamins (). Pigs were ad libitum fed their respective diets and had free access to water throughout the entire experiment.
Table 1. Test dairy products nutrient composition
Table 2. Ingredients and formulated dietary nutrients
The study began after 3 d of acclimation to individual crates and was divided into two experimental periods. During period 1, pigs remained in constant thermoneutral conditions (19 °C; ~46% humidity; temperature-humidity index ≈63)Citation18 for 7 d. During period 2, pigs were exposed to constant HS conditions (32 °C; ~26% humidity; temperature-humidity index ≈76) for 24 h. At the end of period 2, pigs were sacrificed using the captive bolt technique followed by exsanguination. Throughout the experiment, ambient temperature was controlled but humidity was not governed, and both parameters were monitored and recorded every 30 min by data loggers (EL-USB-2-LCD, Lascar). Temperature-humidity index ranged between 58–65 and 75–79 during period 1 and 2, respectively.
During period 1, body temperature indices (respiration rate and rectal temperature) were obtained four times a day (0800, 1200, 1600 and 2000 h) and condensed into daily averages and period average. During period 2, temperature indices were obtained at 0, 4, 8, 12 and 24 h relative to the initiation of HS. Respiration rate was determined by counting flank movements and rectal temperature was measured using a digital thermometer (V901H, Vicks®). Individual feed intake was recorded daily as-fed throughout the experiment. Body weights were collected at the beginning of each period and immediately prior to sacrifice.
Blood was obtained (K2EDTA blood tubes, BD vacutainers®, cat# 367861) on day 6 of period 1 (24 h prior to initiation of HS) and at sacrifice and kept in ice until processing. Plasma was harvested by centrifugation at 1300 x g and stored at -80 °C for later analysis. Whole sections from both the proximal ileum (1.5 m proximal to the ileocecal junction) and distal colon (0.5 m proximal to the rectum) were harvested immediately following euthanasia. Intestinal segments were processed as previously described.Citation8 Due to logistical constraints in sample collection and analysis, groups of 4 pigs (1 pig per treatment) were sacrificed twice a day (8 pig/d) for 4 consecutive days. Each group of 4 pigs was considered a “set” for statistical purposes. The timing of each measured variable was similar among sets.
Ex vivo measures of intestinal integrity
Ileal and colonic segments of each animal were mounted into modified Ussing chambers (Physiological Instruments) for determination of intestinal integrity. The TER and FITC-Dextran APP (4.4 kDa; Sigma®, cat# FD4) were measured and calculated as previously described by Pearce et al.Citation7 Chamber slides had a surface area of 0.7 cm2 and all readings were corrected to a 1 cm2 surface.
Blood parameters analyses
Plasma L-lactate and D-lactate concentrations were measured enzymatically using commercially available kits (Biomedical Research Service Center). Plasma LBP concentrations were determined using an ELISA kit (Hycult® biotech, cat# HK503). The intra- and inter-assay coefficients of variation were 6.6 and 5.1% for L-lactate, 1.5 and 2.3% for D-lactate, and 8.7 and 22.2% for LBP.
Statistical analyses
Data are reported as LSmeans and considered significant if P ≤ 0.05 and a tendency if 0.05 < P ≤ 0.10. Variables with single measurements were statistically analyzed using the PROC GLM procedure of SAS version 9.2 (SAS Inst. Inc.). The model included treatment and set as fixed effects. For a given variable, when an initial measurement (at the beginning of the period or during period 1) was available it was used as a covariate.
Variables with multiple measurements were analyzed using the PROC MIXED procedure of SAS. Each animal’s respective parameter was analyzed using repeated measures with an auto regressive covariance structure. The repeated effect was hour after initiation of HS (rectal temperature and respiration rate) or period (blood parameters). The model included treatment, the repeated effect, set, and treatment by the repeated effect interaction as fixed effects; and covariate when available. For both procedures, set and the covariate were only kept in the model if their P ≤ 0.20. Contrasts were performed to estimate differences between each dietary treatment and the Ct-fed pigs. For each variable, residuals distribution was tested for normality and logarithmic transformation was performed when necessary.
Results
During period 1, there were no differences in body temperature indices () among diets. As expected during period 2, rectal temperature and respiration rate markedly increased by 0.85 °C and ~3-fold, respectively (P < 0.01; ). There was a time effect (P ≤ 0.05; ) for both indices as rectal temperature and respiration rate peaked at 12 and 4 h post-HS initiation, respectively; however, no dietary treatment differences in these variables were detected. Both rectal temperature and respiration rate remained markedly increased (0.6 °C and 3-fold, respectively) compared with period 1 (.A and 1.B).
Figure 1. Effects of feeding diets containing no test product (Ct), or 80% milk whey protein (WP) + 20% colostral whey protein (CWP; WP80), 98% WP + 2% CWP (WP98), 100% WP (WP100) test products on (A) rectal temperature and (B) respiratory rate of pigs exposed to constant heat stress conditions (32 °C) for 24 h. a,b,c Represent differences between hours of heat stress (P ≤ 0.05). *Represents the average values during period 1.
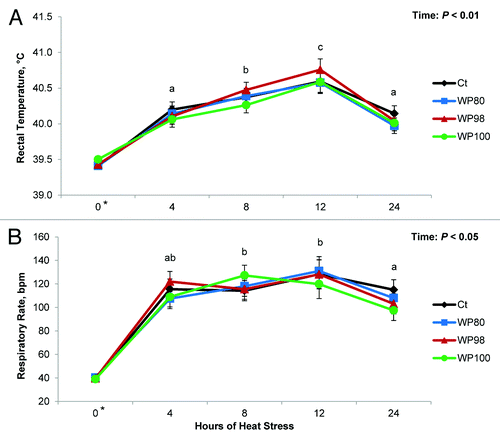
During period 1, feed intake (2.1 kg/d), average daily gain (0.74 kg/d) and gain to feed ratio (0.35) were not different between diets (). During period 2 (after 24 h of HS), pigs in all treatments similarly reduced their intake and lost body weight (44% and -0.5 kg, respectively; ).
Table 3. Effects of dietary dairy products and environmental conditions on production parameters in pigs
There was a diet effect on ileal TER, as it was decreased (P ≤ 0.05) and tended to be decreased (P ≤ 0.10) in WP80 and WP100-fed pigs (37 and 27%, respectively; ), compared with controls. Ileal TER did not differ between WP98 and Ct-fed pigs. There were no dietary treatment differences on ileal FITC-Dextran APP or any measure of colonic integrity ().
Table 4. Effects of dietary dairy products on intestinal permeability parameters in 24 h heat-stressed pigs
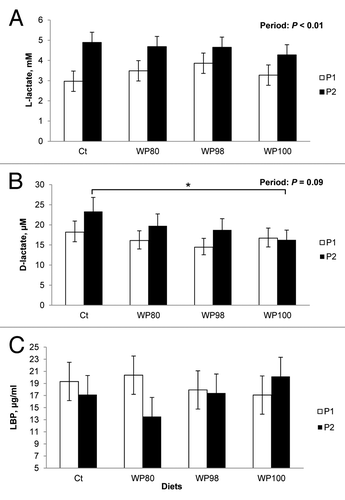
Both plasma L-lactate and D-lactate concentration increased (P < 0.01; 36%) and tended to increase (P = 0.09; 19%), respectively from period 1 to period 2, but the magnitude of the response was similar among dietary treatments (.A and 2.B). During period 2 and using period 1 as a covariate, plasma D-lactate was decreased in WP100-fed pigs (P ≤ 0.05; 29%; Figure 2.C), when compared with Ct-fed pigs. Neither dietary treatment nor period had an effect on plasma LBP concentration (Fig. 2.C).
Figure 2. Effects of feeding diets containing no test product (Ct), or 80% milk whey protein (WP) + 20% colostral whey protein (CWP; WP80), 98% WP + 2% CWP (WP98), 100% WP (WP100) test products on plasma (A) L-lactate, (B) D-lactate, and (C) lipopolysaccharide binding protein (LBP) concentrations of pigs during period 1 (P1; thermoneutral conditions: 19 °C; ~46% humidity) and at the end of period 2 (P2; heat stress conditions for 24 h: 32 °C; ~26% humidity). *Represents differences between treatments during period 2 with period 1 as a covariate.
Discussion
Heat stress negatively affects animal agriculture by reducing growth and reproductive performance and jeopardizing animal welfare. Heat-stressed animals redistribute blood to the periphery in an attempt to maximize radiant heat dissipation.Citation5 Subsequent visceral vasoconstriction leads to intestinal hypoxia in addition to hyperthermia.Citation6,Citation9 As demonstrated by the early upregulation of heat shock proteins during hyperthermia,Citation19 enterocytes are extremely sensitive to oxygen and nutrient restriction,Citation20 resulting in ATP depletion, and increased oxidative and nitrosative stress.Citation21 Ultimately, HS causes marked morphological changes, tight junction disruption, and reduced intestinal barrier function.Citation5 Increased intestinal permeability during HS, elevates portal and systemic blood LPS concentration,Citation7,Citation21 which might mediate some of the negative effects of HS on animal production.Citation22 Interestingly, dairy products have improved intestinal barrier function in a variety of models. Further, we have recently demonstrated that dietary supplementation with zinc partially ameliorated the effects of HS on intestinal permeability in pigs,Citation8 demonstrating that nutritional management is a feasible mitigation strategy for environmental hyperthermia. Thus, we hypothesized that dietary bovine WP and CWP would alleviate the decrease in intestinal integrity observed in pigs during HS.
Contrary to our hypothesis and in disagreement with the literature, feeding WP/CWP did not improve direct measures (i.e., TER and FITC-Dextran APP) of ileal or colonic permeability. Playford et al. demonstrated that dairy product supplementation ameliorates the effects of non-steroidal anti-inflammatory drugs on the gastro-intestinal tract in rodents (i.e., WP and CWP) and humans (i.e., bovine colostrum and milk).Citation12,Citation13 In addition, local treatment with CWP improved symptoms and histological scores of ulcerative colitis patients.Citation14 In vitro studies have also shown the beneficial effects of bovine colostrum and goat milk on TER in response to a tight junction disruptor.Citation15 Finally, dairy products improved intestinal integrity in heat-stressed ratsCitation15 and in an in vitro model of hyperthermia.Citation16 The mechanism by which dairy products may protect intestinal health is not fully elucidated. Both bovine WP and CWP are rich in antimicrobial proteins (e.g., glucomacropeptides, lactoferrin), immunoglobulins, growth factors (transforming growth factor-β), and specific amino acids (glutamine, cysteine, and threonine).Citation23 However, the composition of these products is highly variable depending on the origin (e.g., breed, alimentation and health status), the time of collection, and the post-collection processing; making difficult to identify the bioactive constituents responsible for their positive effects.Citation24 With regard to intestinal health, several mechanisms of action have been attributed to dairy products, including upregulation of heat-shock proteinsCitation16 and tight junction proteins,Citation25 or the increase in mucin production.Citation26 Consequently, there appear to be a variety of mechanisms by which dietary dairy products can reduce gut “leakiness.”
Reasons for the lack of a protective response to our dietary treatments are not clear. In the present study, pigs were severely heat-stressed (constant 32 °C without a thermal recovery period during the night), as demonstrated by a marked increase in all the body temperature indices (rectal temperature = +0.85 °C, respiration rate = +76 breath/min) and sharp decrease in feed intake (44%). We hypothesize that the severe HS may have blunted the potential beneficial effects of WP and CWP on intestinal health. Whether or not mild and/or cyclical HS conditions (more similar to natural heat stress events), would allow for dairy products to express their improvement on intestinal permeability variables remains of interest. Not only were the products in the current study ineffective, feeding WP80 (the diet with the highest content in CWP: 20%) actually increased ileal permeability. This is not unprecedented as it agrees with a human report, where subjects supplemented with bovine colostrum had increased in intestinal permeability after a standardized exercise program compared with controls and individuals receiving WP.Citation27
We recognize that our experimental design (with no thermoneutral control treatment) makes it difficult to directly demonstrate that the gut was actually compromised by HS. However, utilizing a similar heat load and type of pigs (i.e., genetics, body weight, gender) we have repeatedly reported that direct intestinal measurements of gut integrity deteriorated after 24 h of thermal exposure,Citation6,Citation7 and circulating LPS increased.Citation28 In addition, in a similar experimental design we have reported an increase in oxidative stress parameters in skeletal muscle, ostensibly the consequence of heat-stressed induced endotoxemia.Citation29 In the current experiment we measured blood biomarkers of leaky gut (i.e., D- and L-lactate and LBP) in both period 1 and 2. D-lactate is a product of microbial metabolism, so its presence in blood likely indicates an increase in gut leakiness.Citation30,Citation31 As expected, D-lactate increased from period 1 to period 2, confirming that the intestinal barrier function was compromised after 24 h of HS. Lipopolysaccharide binding protein is an acute phase protein that binds LPS and mediates its interaction with toll-like receptor 4,Citation32 resulting in the activation of the innate immune response. Interestingly, high circulating concentrations of LBP inhibit LPS-induced inflammation.Citation33 We have previously reported that plasma LBP decreases as HS progresses (1–12 h) and intestinal integrity deteriorates.Citation28 Reasons why we did not observe a period effect on plasma LBP concentrations remain unknown. Noteworthy, at the end of period 2, WP100-fed pigs had lower plasma D-lactate concentration compared with Ct-fed pigs and a numerical increase in plasma LBP from period 1 to period 2, which has been previously associated with decreased circulating LPS.Citation28 In agreement, WP100-fed pigs had a numerical improvement in intestinal integrity (increased TER and decreased FITC-dextran APP) at the colon level, where most of the microflora is located, which might explain the aforementioned changes in plasma parameters.
L-lactate is the product of anaerobic glycolysis. The rationale to utilize this parameter as a biomarker of intestinal integrity is based on the release of intracellular L-lactate by damaged intestinal cells.Citation31 However, a similar phenomenon occurs in non-intestinal cells, which makes L-lactate a nonspecific measurement of cellular damage. Our baseline (period 1) L-lactate levels are higher than those previously reported,Citation31,Citation34 however, our blood collection method (jugular venipuncture), which requires physical constraint, could have triggered a stress response which is known to increase L-lactate levels.Citation35 Nevertheless, this procedure was consistently performed in both periods. Notably, the increase in plasma L-lactate has been repeatedly observed during HS.Citation22 Our recent data suggests that complete glucose oxidation in skeletal muscle is decreased during HS, thus L-lactate is produced via aerobic glycolysis.Citation22 Therefore, increased circulating L-lactate might be the result of metabolic adaptations to HS, resembling the Warburg effect employed by cancerous cells.Citation36 Whether the increase in circulating L-lactate from period 1 to period 2 is a result of cellular damage (presumably intestinal damage) or altered systemic glucose metabolism remains to be elucidated.
Heat stress is one of the largest impediments to efficient animal production. A hallmark of heat-stressed animals is a compromised intestinal integrity, and the subsequent endotoxemia might contribute to reduced animal productivity. We have herein demonstrated that feeding a combination of bovine WP and CWP in the tested proportions failed to ameliorate the effects of severe and constant HS on intestinal integrity of pigs. Whether milder and cyclical HS conditions (resembling those in commercial settings) and different levels of inclusion of the test dairy products would allow for improvement on intestinal health remains of interest.
| Abbreviations: | ||
| CWP | = | colostral whey protein |
| FITC-Dextran APP | = | fluorescein isothiocyanate labeled dextran apparent permeability coefficient |
| HS | = | heat stress |
| LBP | = | lipopolysaccharide binding protein |
| LPS | = | lipopolysaccharide |
| TER | = | transepithelial electrical resistance |
| WP | = | milk whey protein |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Results described here within were supported by the Midwest Dairy Association, the Fulbright Graduate Student Program for Spaniards from the Commission for Cultural, Educational and Scientific Exchange between the United States of America and Spain; and the Agricultural and Food Research Initiative Competitive Grant no. 2011–67003–30007 from the USDA National Institute of Food and Agriculture. Any opinions, findings, conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of any of the above mentioned organizations. The authors express their appreciation to Anna Gabler, Martha Jeffrey, Samantha Lei, Dana Van Sambeek, Amir Nayeri, and Jacob Schulte for their assistance in the live and laboratory phases of the experiment.
References
- St-Pierre NR, Cobanov B, Schnitkey G. Economic losses from heat stress by US livestock industries. J Dairy Sci 2003; 86:E52 - 77; http://dx.doi.org/10.3168/jds.S0022-0302(03)74040-5
- Luber G, McGeehin M. Climate change and extreme heat events. Am J Prev Med 2008; 35:429 - 35; http://dx.doi.org/10.1016/j.amepre.2008.08.021; PMID: 18929969
- Brown-Brandl TM, Nienaber JA, Xin H, Gates RS. A literature review of swine heat production. Trans. Am Soc. Agric Eng 2004; 47:259 - 70
- Rhoads RP, Baumgard LH, Suagee JK, Sanders SR. Nutritional interventions to alleviate the negative consequences of heat stress. Adv Nutr 2013; 4:267 - 76; http://dx.doi.org/10.3945/an.112.003376; PMID: 23674792
- Lambert GP, Gisolfi CV, Berg DJ, Moseley PL, Oberley LW, Kregel KC. Selected contribution: Hyperthermia-induced intestinal permeability and the role of oxidative and nitrosative stress. J Appl Physiol (1985) 2002; 92:1750 - 61, discussion 1749; PMID: 11896046
- Pearce SC, Mani V, Weber TE, Rhoads RP, Patience JF, Baumgard LH, Gabler NK. Heat stress and reduced plane of nutrition decreases intestinal integrity and function in pigs. J Anim Sci 2013; 91:5183 - 93; http://dx.doi.org/10.2527/jas.2013-6759; PMID: 23989867
- Pearce SC, Mani V, Boddicker RL, Johnson JS, Weber TE, Ross JW, Rhoads RP, Baumgard LH, Gabler NK. Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS One 2013; 8:e70215; http://dx.doi.org/10.1371/journal.pone.0070215; PMID: 23936392
- Sanz Fernandez MV, Pearce SC, Gabler NK, Patience JF, Wilson ME, Socha MT, Torrison JL, Rhoads RP, Baumgard LH. Effects of supplemental zinc amino acid complex on gut integrity in heat-stressed growing pigs. Animal 2014; 8:43 - 50; http://dx.doi.org/10.1017/S1751731113001961; PMID: 24229744
- Hall DM, Baumgardner KR, Oberley TD, Gisolfi CV. Splanchnic tissues undergo hypoxic stress during whole body hyperthermia. Am J Physiol 1999; 276:G1195 - 203; PMID: 10330010
- Bynum G, Brown J, Dubose D, Marsili M, Leav I, Pistole TG, Hamlet M, LeMaire M, Caleb B. Increased survival in experimental dog heatstroke after reduction of gut flora. Aviat Space Environ Med 1979; 50:816 - 9; PMID: 496751
- Gathiram P, Wells MT, Brock-Utne JG, Gaffin SL. Antilipopolysaccharide improves survival in primates subjected to heat stroke. Circ Shock 1987; 23:157 - 64; PMID: 3427771
- Playford RJ, Floyd DN, Macdonald CE, Calnan DP, Adenekan RO, Johnson W, Goodlad RA, Marchbank T. Bovine colostrum is a health food supplement which prevents NSAID induced gut damage. Gut 1999; 44:653 - 8; http://dx.doi.org/10.1136/gut.44.5.653; PMID: 10205201
- Playford RJ, MacDonald CE, Calnan DP, Floyd DN, Podas T, Johnson W, Wicks AC, Bashir O, Marchbank T. Co-administration of the health food supplement, bovine colostrum, reduces the acute non-steroidal anti-inflammatory drug-induced increase in intestinal permeability. Clin Sci (Lond) 2001; 100:627 - 33; http://dx.doi.org/10.1042/CS20010015; PMID: 11352778
- Khan Z, Macdonald C, Wicks AC, Holt MP, Floyd D, Ghosh S, Wright NA, Playford RJ. Use of the ‘nutriceutical’, bovine colostrum, for the treatment of distal colitis: results from an initial study. Aliment Pharmacol Ther 2002; 16:1917 - 22; http://dx.doi.org/10.1046/j.1365-2036.2002.01354.x; PMID: 12390100
- Prosser C, Stelwagen K, Cummins R, Guerin P, Gill N, Milne C. Reduction in heat-induced gastrointestinal hyperpermeability in rats by bovine colostrum and goat milk powders. J Appl Physiol (1985) 2004; 96:650 - 4; http://dx.doi.org/10.1152/japplphysiol.00295.2003; PMID: 14527963
- Marchbank T, Davison G, Oakes JR, Ghatei MA, Patterson M, Moyer MP, Playford RJ. The nutriceutical bovine colostrum truncates the increase in gut permeability caused by heavy exercise in athletes. Am J Physiol Gastrointest Liver Physiol 2011; 300:G477 - 84; http://dx.doi.org/10.1152/ajpgi.00281.2010; PMID: 21148400
- National Research Council. Nutrient requirements of swine, 10th edition. National Academy Press, 1998.
- Federation of Animal Sciences Societies. Guide for the care and use of agricultural animals in research and teaching. 3rd edition. www.fass.org, 2010.
- Flanagan SW, Ryan AJ, Gisolfi CV, Moseley PL. Tissue-specific HSP70 response in animals undergoing heat stress. Am J Physiol 1995; 268:R28 - 32; PMID: 7840333
- Rollwagen FM, Madhavan S, Singh A, Li YY, Wolcott K, Maheshwari R. IL-6 protects enterocytes from hypoxia-induced apoptosis by induction of bcl-2 mRNA and reduction of fas mRNA. Biochem Biophys Res Commun 2006; 347:1094 - 8; http://dx.doi.org/10.1016/j.bbrc.2006.07.016; PMID: 16870148
- Hall DM, Buettner GR, Oberley LW, Xu L, Matthes RD, Gisolfi CV. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am J Physiol Heart Circ Physiol 2001; 280:H509 - 21; PMID: 11158946
- Baumgard LH, Rhoads RP. Effects of Heat Stress on Postabsorptive Metabolism and Energetics. Annu Rev Anim Biosci 2013; 1:311 - 37; http://dx.doi.org/10.1146/annurev-animal-031412-103644
- Krissansen GW. Emerging health properties of whey proteins and their clinical implications. J Am Coll Nutr 2007; 26:713S - 23S; http://dx.doi.org/10.1080/07315724.2007.10719652; PMID: 18187438
- Kelly GS. Bovine colostrums: a review of clinical uses. Altern Med Rev 2003; 8:378 - 94; PMID: 14653766
- Hering NA, Andres S, Fromm A, van Tol EA, Amasheh M, Mankertz J, Fromm M, Schulzke JD. Transforming growth factor-β, a whey protein component, strengthens the intestinal barrier by upregulating claudin-4 in HT-29/B6 cells. J Nutr 2011; 141:783 - 9; http://dx.doi.org/10.3945/jn.110.137588; PMID: 21430244
- Sprong RC, Schonewille AJ, van der Meer R. Dietary cheese whey protein protects rats against mild dextran sulfate sodium-induced colitis: role of mucin and microbiota. J Dairy Sci 2010; 93:1364 - 71; http://dx.doi.org/10.3168/jds.2009-2397; PMID: 20338413
- Buckley JD, Butler RN, Southcott E, Brinkworth GD. Bovine colostrum supplementation during running training increases intestinal permeability. Nutrients 2009; 1:224 - 34; http://dx.doi.org/10.3390/nu1020224; PMID: 22253980
- Sanz Fernandez MV, Pearce SC, Gabler NK, Patience JF, Wilson ME, Socha MT, Torrison JL, Rhoads RP, Baumgard LH. Effects of supplemental zinc amino acid complex on gut integrity in heat-stressed growing pigs. Animal 2014; 8:43 - 50; http://dx.doi.org/10.1017/S1751731113001961; PMID: 24229744
- Rosado Montilla SI, Johnson TP, Pearce SC, Gardan-Salmon D, Gabler NK, Ross JW, Rhoads RP, Baumgard LH, Lonergan SM, Selsby JT. Heat stress causes oxidative stress but not inflammatory signaling in porcine skeletal muscle. Temperature 2014; 1:1 - 9
- Ewaschuk JB, Naylor JM, Zello GA. D-lactate in human and ruminant metabolism. J Nutr 2005; 135:1619 - 25; PMID: 15987839
- Nielsen C, Mortensen FV, Erlandsen EJ, Lindholt JS. L- and D-lactate as biomarkers of arterial-induced intestinal ischemia: an experimental study in pigs. Int J Surg 2012; 10:296 - 300; http://dx.doi.org/10.1016/j.ijsu.2012.05.003; PMID: 22595440
- Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine 2008; 42:145 - 51; http://dx.doi.org/10.1016/j.cyto.2008.01.006; PMID: 18304834
- Hamann L, Alexander C, Stamme C, Zähringer U, Schumann RR. Acute-phase concentrations of lipopolysaccharide (LPS)-binding protein inhibit innate immune cell activation by different LPS chemotypes via different mechanisms. Infect Immun 2005; 73:193 - 200; http://dx.doi.org/10.1128/IAI.73.1.193-200.2005; PMID: 15618154
- Rixen D, Raum M, Holzgraefe B, Schäfer U, Hess S, Tenhunen J, Tuomisto L, Neugebauer EA, Shock and Trauma Study Group. Local lactate and histamine changes in small bowel circulation measured by microdialysis in pig hemorrhagic shock. Shock 2002; 18:355 - 9; http://dx.doi.org/10.1097/00024382-200210000-00011; PMID: 12392280
- Hemsworth PH, Barnett JL, Hofmeyr C, Coleman GJ, Dowling S, Boyce J. The effects of fear of humans and pre-slaughter handling on the meat quality of pigs. Aust J Agric Res 2002; 53:493 - 501; http://dx.doi.org/10.1071/AR01098
- Kim JW, Dang CV. Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res 2006; 66:8927 - 30; http://dx.doi.org/10.1158/0008-5472.CAN-06-1501; PMID: 16982728
