Abstract
Destabilization of cell-cell contacts involved in the maintenance of endothelial barrier function can lead to increased endothelial permeability. This increase in endothelial permeability results in an anarchical movement of fluid, solutes and cells outside the vasculature and into the surrounding tissues, thereby contributing to various diseases such as stroke or pulmonary edema. Thus, a better understanding of the molecular mechanisms regulating endothelial cell junction integrity is required for developing new therapies for these diseases. In this review, we describe the mechanotransduction mechanism at the basis of adherens junction strengthening at endothelial cell-cell contacts. More particularly, we report on the emerging role of α-catenin and EPLIN that act as a mechanotransmitter of myosin-IIgenerated traction forces. The interplay between α-catenin, EPLIN and the myosin-II machinery initiates the junctional recruitment of vinculin and α-actinin leading to a drastic remodeling of the actin cytoskeleton and to cortical actin ring reshaping. The pathways initiated by tyrosine phosphorylation of VE-cadherin at the basis of endothelial cell–cell junction remodeling is also reported, as it may be interrelated to α-catenin/ EPLIN-mediated mechanotransduction mechanisms. We also describe the junctional mechanosensory complex composed of PECAM-1, VE-cadherin and VEGFR2 that is able to transmit signaling pathway under the onset of shear stress. This mechanosensing mechanism, involved in the earliest events promoting atherogenesis, is required for endothelial cell alignment along flow direction.
Introduction to Mechanotransduction
It is now well known that mechanical forces can be converted into biochemical signals by mechanisms generally designated as mechanotransduction. These mechanisms have critical roles in the maintenance of the integrity of mechanically stressed tissues such as blood vessels, muscles, bones and cartilage. At the cellular level, mechanotransduction can modulate diverse functions including protein synthesis, adhesion, proliferation, differentiation, viability and apoptosis. Defects in cellular mechanotransduction can contribute to various human diseases. Thus, genetic mutations found to interfere with normal mechanotransduction and cellular sensibility to mechanical stress are involved in a wide spectrum of diseases ranging from loss of hearing,Citation1 lung dysfunction,Citation2,Citation3 muscular dystrophiesCitation4 and cancer.Citation5 A common denominator of many of these diseases is the disruption in the force transmission between the actin cytoskeleton and the extracellular matrix (ECM) or cell–cell junctions.
Identifying the molecular basis involved in normal or defective mechanotransduction will shed light on the underlying disease mechanisms and normal cellular functions and could lead to new therapeutic approaches for these diseases.
Mechanotransduction in Endothelium
Due to heart contraction, blood circulates through the vasculature in a pulsatile fashion thus submitting vessels to mechanical forces resulting both from shear stress and smooth muscle cell contractility.Citation6,Citation7 At the inner wall of vessels, the vascular endothelium senses and responds to these stimuli. To adapt itself to such constraints, the endothelium undergoes remodeling and initiates a series of signals. These events are accentuated in the pulmonary vasculature because in lungs the vessel walls are submitted, in addition to blood circulation stress, to the rhythmic respiratory activity.Citation8
Mechanical forces emerging from the blood flow have a great impact on the physiopathology of the vascular tree, in particular on the cardiovascular system. While laminar flow occurs in straight vessels and produces a steady shear stress on endothelial cells, at bends or bifurcations, shear stress fluctuates with the creation of turbulence.Citation9 These drastic changes of local hemodynamics in curved and branched areas of the arteries promote atherosclerosis. Indeed, regions of the vasculature exposed to disturbed blood flow coincide with specific sites where atherosclerotic plaques are preferentially formed. Consequently, the capacity of endothelial cells to sense and respond to blood flow is critical in the development of cardiovascular pathologies such as atherosclerosis.
In the vessel wall, most of the responses to flow only affect endothelial cells indicating that these cells possess specific mechanotransducers that convert mechanical forces into biological responses. The precise mechanisms by which forces are transduced to biochemical signals inside endothelial cells are poorly understood. Nevertheless, it has been proposed that shear stress-mediated forces are transmitted from the apical surface of endothelial cells by the cytoskeleton to sites of attachment at cell–cell and cell–matrix adhesions.Citation10 This suggested that focal adhesion (FA), tight junction (TJ) and adherens junction (AJ) adhesive structures are involved in mechanotransduction ().
Figure 1. Side-view of cells adhering to ECM via FA and to each other via TJ and AJ. Tangential (○) and centripetal (→) forces exerted by actomyosin
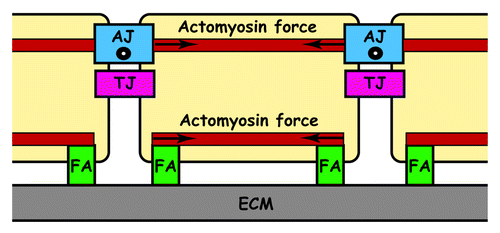
IntegrinsCitation11 at FAs, occludin at TJsCitation12 and VE-cadherin at AJs have been proposed as putative mechanotransducers able to sense blood flow.Citation13 Activation of these mechanotransducers initiates several signaling pathways affecting cytoskeletal structures. In particular, laminar flow initiates the Rho GTPase pathway that promotes the alignment of actin fibers along the flow axis and assembly of adherensCitation11,Citation14-Citation17 and tight junctions.Citation18 By contrast, perturbed flow leads to a disorganized actin cytoskeleton and disassembly of adherens junctions.Citation17,Citation19
Endothelial cells can sense the type of shear flow and consequently align their cytoskeleton, thus modulating their traction forces. This modification of cytoskeletal tension subsequently affects the intercellular forces that regulate the assembly/disassembly of cell-cell contacts. Thus, laminar flow increases cytoskeletal tension that, in turn, promotes cell-cell contacts, while disturbed flow weakens cellular forces leading to a dislocation of cell-cell contacts.Citation17
Mechanisms at the Basis of Force Transduction
Mechanotransduction at FAs
FAs constitute discrete areas located at the ventral surface of cells that interconnect cells with the ECM. These large multiprotein complexes are composed of integrins, a family of adhesive receptors able to interact with proteins of the ECM and to recruit intracellular partners. Integrins are known to be mechanotransducers critically involved in force-sensing processes exerting their action between the ECM and the contractile actomyosin cytoskeleton.Citation20 The dynamic crosstalk between the actin cytoskeleton and the ECM plays a major role in cell movement, cell adhesion and matrix remodeling.
On the cytoplasmic side of FAs, integrins can interact via their intracellular domains with at least 12 adaptor proteins.Citation21 Among them, talin, tensin, filamin and α-actinin can elaborate links to the actin cytoskeleton. These preliminary interactions with the cytoskeleton are reinforced by a second and third tier of regulatory proteins that stabilize the adhesome network. This vision was recently confirmed by P. Kanchanawong and coworkers, who established that FAs possess a stratified architecture in which integrins and actin are vertically separated by an adhesive core composed of multiple protein-specific strata that contain more than 150 proteins collectively designated as FA adhesome.Citation22
Mechanotransduction at TJs
TJs link cell membranes closely together thus creating a barrier that is impermeable to fluid.Citation23,Citation24 More specifically, at the blood–brain barrier, TJs play a key role by limiting the diffusion of substrates from blood to brain.Citation25 The proteins constituting TJs include occludin, claudin family members, junctional adhesion molecules (JAM) and cytosolic scaffolding proteins such as the zonula occludens family members (ZO) that link TJ proteins to each other and the actin cytoskeleton.Citation24,Citation25
Because they are intimately linked to the hemodynamically responsible actin cytoskeleton,Citation26 TJs are sensitive to shear stress. For instance, in vascular endothelial cells, the expression of TJ proteins is subjected to regulation by hemodynamic forcesCitation12,Citation27-Citation29 thereby affecting endothelial barrier permeability. According to DeMaio et al., shear stress reduces occludin mRNA and protein expression in parallel with an increase in tyrosine phosphorylation and endothelial permeability.Citation12 By contrast, Conklin et al. have observed a shear-stress upregulation of occluding mRNA.Citation28 In fact, alterations in TJ protein expression and linkage between TJ proteins and the actin cytoskeleton in part govern permeability and endothelial barrier function.Citation30 However, further investigation is still required to improve our understanding of the contribution of shear stress to the modulation of TJ protein composition and consequently to endothelial barrier permeability.
Mechanotransduction at AJs
At cell-cell contacts, AJs are adhesion complexes that resist dissociating forces and also transmit forces to adjacent cells making important contributions to embryogenesis and tissue homeostasis.Citation31,Citation32 For instance, they are remodeled dynamically during morphogenesis, tissue regeneration or repair, events during which actin filament association with AJs plays a crucial role.
The core of AJs is composed of cadherins, a family of transmembrane Ca++-dependent adhesion molecules. Cadherins are able to support large forces. Indeed, suspended cells expressing cadherins adhere to each other and require varying forces for their separation, depending on the cadherin type.Citation33,Citation34 In fact, as demonstrated by Le Duc et al. using nanomechanical measurements, cadherin complexes also function as mechanosensors able to transmit forces.Citation35 Thus, AJs transfer to adjoining cells cellular contractility forces generated by the actomyosin machinery.Citation31,Citation36-Citation38 This transmission results from the multiple couplings existing between cadherins and the actomyosin cytoskeleton.
Despite the complexity of FA and AJ adhesive structures, an understanding of the functioning of FA and AJ mechanosensory machineries is now emerging. Forces applied at FAs have been shown to promote integrin-based adhesion assembly and maturation by inducing sequential recruitment of various regulatory proteins,Citation39,Citation40 which enables transmission of tension between the cytoskeleton and integrins.Citation41 Depending on the tension exerted by the cytoskeleton, FAs undergo reinforcement recognizable by an increase in integrin and vinculin density in cells exhibiting no intercellular contact.Citation42 Similarly, AJs are reinforced in response to external tension,Citation43 which leads to an increase of cadherin and vinculin density.Citation35,Citation44 The FA-and AJ-mediated mechanotransduction mechanism appears in part governed by force-induced conformational switches in the structures of scaffolding proteins. These conformational changes unmask cryptic binding sites for additional proteins.Citation39
Mechanotransduction has been essentially studied at FAs. Recently, more attention has been brought to mechanotransduction involved at AJs. Hereafter, we focus our attention on the mechanotransduction mechanism specifically exerted at interendothelial cell–cell junctions.
Modulation of the Endothelial Barrier via Mechanotransduction Governed by the VEcadherin Complex
Endothelial cell–cell junctions include tight, gap and adherens junctions that are mediated by occludin/claudins, connexins and VE-cadherin, respectively.Citation45 Among these adhesive structures, AJs are the most cohesive and dynamic and also the most important for transmitting mechanical signals directly to the actin cytoskeleton.
At endothelial AJs, a cell-specific member of the cadherin protein family, VE-cadherin (VE-cad), is expressed that holds together endothelial cells and plays a crucial role in the maintenance and restoration of endothelium integrity and in the regulation of vessel permeability.Citation46-Citation48
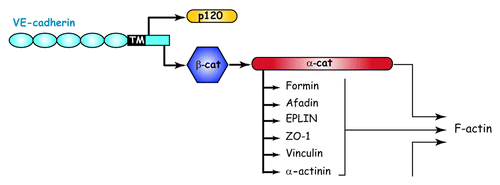
Similarly to classical cadherins, the VE-cadherin extracellular domain mediates homophilic/homotypic interactions. To maintain junction integrity, these interactions must be strengthened by intracellular connections to the actin cytoskeleton of contacting cells. Thus, the C-terminus of the cytoplasmic domain of VE-cadherin binds, in a mutually exclusive fashion, β-catenin or γ-catenin that in turn recruits α-catenin (). α-catenin is indispensable for cadherin-mediated cell–cell adhesion, because without it, adherens junctions are disrupted and the association of actin filaments to the cadherin–catenin complex is completely lost.Citation49 Until recently, it was widely accepted that α-catenin strengthens cadherin-mediated adhesion by directly promoting anchorage to the actin cytoskeleton. Nevertheless, in 2005, this concept was challenged by studies demonstrating that α-catenin is unable to bind simultaneously β-catenin and actin.Citation50,Citation51 Altogether, these data support the notion that additional intracellular partners are required for connecting cadherin-bound αcatenin to the actin cytoskeleton.
Figure 2. Organization and cytoskeletal relationship of cadherin-catenin complex. Adapted from reference Citation93.
Endothelial barrier function can be reinforced by strengthening connections between the AJ structures and the actin cytoskeleton. As seen hereafter, mechanotransduction mechanisms operating at the AJ interface participate in the elaboration/stiffening of the cortical actin ring, thus orchestrating endothelial barrier reinforcement. Recently, these in vitro observations were confirmed in vivo by Vestweber’s group, who generated mice with strongly stabilized endothelial junctions by genetically replacing VE-cadherin by a VE-cadherin-α-catenin fusion construct. Such mice exhibit strong resistance to induction of vascular leaks by VEGF and histamine and, consequently, the recruitment of neutrophils and leukocytes at inflammatory sites is strongly inhibited. This can be explained by the fact that the fusion protein VEcadherin–α-catenin associates with the actin cytoskeleton with an enhanced capability compared with wild-type VE-cadherin.Citation52
Contribution of α-catenin to mechanotransduction at interendothelial AJs
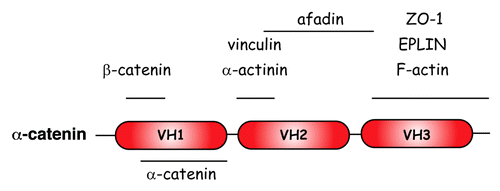
The molecule of α-catenin possesses three vinculin homology (VH) domains designated as VH1, 2 and 3 ().Citation53 Each of them has the capacity to interact with a plethora of actin-binding proteins. Thus, vinculin,Citation54 α-actinin,Citation55,Citation56 afadinCitation55 and forminCitation57 bind to the central VH2 domain, whereas EPLINCitation58 and ZO-1Citation59 bind to the C-terminal VH3 domain of α-catenin. Furthermore, the N-terminal VH1 domain contains the β/γ-cateninbinding domain,Citation60,Citation61 whereas the C-terminal VH3 domain contains the α-catenin actin-binding domain.Citation55,Citation62
Figure 3. Schematic representation of the a-catenin molecule and its binding capacity Adapted from reference Citation53.
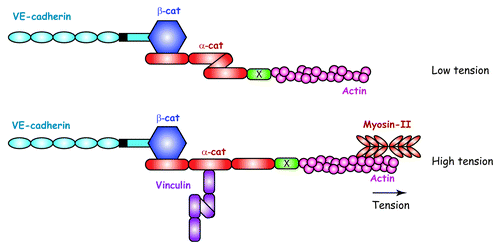
Recently, α-catenin has become the focus of intensive research to unravel the mechanisms at the basis of mechanotransduction at AJs. In this context, it was shown that the overall conformation of α-catenin was altered by forces supplied by myosin II and transmitted through the actin cytoskeleton to AJs between epithelial cells.Citation63,Citation64 These structural changes unmask a vinculin-binding site previously buried in the unstressed molecule of αcatenin. In fact, an inhibitory region within the α-catenin VH2 domain prevents vinculin binding when α-catenin is submitted to low tension. This inhibition is released when αcatenin is subjected to traction forces, as illustrated in .
Figure 4. Force-dependent molecular mechanism at the basis of the junctional recruitment of vinculin by a-catenin. Under low tension, a-catenin exhibits a folded conformation sheltering a buried vinculin-binding site. Tension exerted by actomyosin filaments unfurls a-catenin unmasking a vinculin-binding site.Citation38,Citation54 X represents an a-catenin partner able to transmit actomyosinmediated tension. Adapted from reference Citation54.
Contribution of EPLIN to mechanotransduction at interendothelial AJs
We suspected that in the endothelium, α-catenin also undergoes conformational modifications when subjected to tensional forces. In this context, the question that remains to be solved is how α-catenin can be subjected to actomyosin-generated forces in the endothelium. This may be done by an intermediate actin-binding protein that directly associates with α-catenin (). If expressed in endothelial cells, EPLIN (epithelial protein lost in neoplasm) is an attractive candidate, since this protein is able to bind both α-catenin and actin filaments at epithelial AJs.Citation58
EPLIN belongs to the family of LIM domain proteins as it contains a centrally located LIM domain known to form two closely packed zinc-binding subdomains.Citation65 Additionally, EPLIN exhibits two functional acting-binding sites, one on each side of the centrally located LIM domain, that give it the ability to cross-link and bundle actin filaments.Citation66 In vitro, EPLIN stabilizes actin filaments by preventing their depolymerization and blocks the formation of branched filaments by inhibiting actin nucleation by Arp2/3.Citation66 Based on these properties, EPLIN appears implicated in different actin-related processes such as cell motility and migration, cytokinesis and intercellular junctions.Citation58,Citation67-Citation69 Subsequent in vivo studies confirmed the downregulation of EPLINα in a number of human epithelial cancer cells and tissues, suggesting that the loss of EPLINα could contribute to the transformed phenotype. This can be correlated with the fact that during epithelial-mesenchymal transition induced by EGF, EPLIN becomes phosphorylated before being ubiquitinated and degraded.Citation70 This indicates that EPLIN may act as a tumor suppressor.Citation67
We have establishedCitation71 that EPLIN is also expressed in endothelial cells at cell-cell junctions where it co-localizes with α-catenin and actin. In fact, EPLIN associates with the VE-cadherin-catenin complex via α-catenin. To establish the role of EPLIN in mechanotransduction at endothelial AJ, its expression was silenced by siRNA and the impact of such an abrogation on vinculin localization was analyzed. Vinculin appeared co-localized with EPLIN at sites where cell-cell junctions are perfectly mature. By contrast a clear delocalization of vinculin from AJ to FA was observed in EPLIN-depleted HUVEC. When force-generated by actomyosin machinery was blocked by an inhibitor of myosin-II activity, vinculin disappears from endothelial AJ and translocates to FA while EPLIN remains junctional. We have also noticed that AJ recruit variable amounts of vinculin according to the tension amplitude exerted by the cytoskeleton. It can be concluded that EPLIN is present at AJ independently of myosin-II contractility while junctional localization of vinculin requires EPLIN and myosin-II activity. By contrast, Taguchi and al, reported that, in epithelial cells, EPLIN localization at AJs appears mechanosensitive.Citation72 In this paper, downregulation of junctional tension was found to completely abolish EPLIN expression at epithelial AJ although vinculin still localized at these junctions. This contradicts our observations made on endothelial cells.Citation71 Further investigations are required to analyze the discrepancy on EPLIN role at AJ observed between endothelial and epithelial cells.
In the endothelium, our data suggested that, EPLIN triggers conformational changes in α-catenin by both directly interacting with VE-cadherin-bound α-catenin and the actin cytoskeleton. Then, the α-catenin-EPLIN link acts as a mechanotransmitter of forces thus promoting vinculin recruitment at interendothelial AJ ().Citation71 In addition, αcatenin-EPLIN based-mechanotransduction mechanism constitutes a way for transmitting precise quantitative, temporal and spatial information required for AJ reinforcement or remodeling at very specific points.
Figure 5. Hypothetic role of the EPLIN-a-catenin transmitter in the recruitment of vinculin at interendothelial AJs. (A) In the absence of EPLIN, the tension exerted by the actomyosin machinery is not transmitted to a-catenin. a-catenin adopts a folded conformation that prevents vinculin binding. (B) The traction exerted by actomyosin machinery is blocked by blebbistatin, an inhibitor of myosin-II. Despite the presence of EPLIN, a-catenin adopts a folded conformation and vinculin is not recruited at the AJ. (C) When the traction exerted by the actomyosin machinery is transmitted through EPLIN, a-catenin unfurls unmasking a vinculin-binding site. This allows vinculin recruitment at endothelial cell–cell junctions. (D) Once recruited at AJ, vinculin adopts an open conformation unmasking potential binding sites for additional actin-binding proteins X.
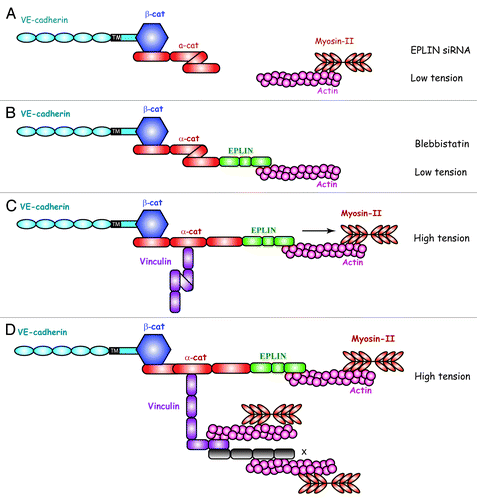
When submitted to tension, the VE-cadherin-α-catenin-EPLIN complex contributes to the strengthening of actin cortical ring and endothelial barrier function. Conversely, reduction of cortical F-actin density disturbs AJ. This was clearly illustrated in vitro using EPLIN-depleted HUVECs in in vitro angiogenesis assays. Indeed, EPLIN-silenced HUVECs kept the ability to form pseudo-vascular networks in matrigel but the capillaries progressively regressed due to numerous breakage events as intercellular tension increased.Citation71
Contribution of vinculin to mechanotransduction at interendothelial AJs
After vinculin linkage to α-catenin at AJs, additional protein components might be recruited locally by tension. Recruitment of these new components might depend on αcatenin-tethered vinculin that may act, in turn, as a mechanosensing protein.
Vinculin molecule consists of head, neck and tail domains. In vitro, vinculin is known to oscillate between an inactive autoinhibited (closed) and active (open) conformation.Citation73 The inactive form results from a high-affinity interaction between the vinculin head and tail domains. Disruption of this intramolecular interaction within vinculin molecule appears to be necessary so as to allow interactions between vinculin and its binding partners.Citation74 The pathway involved in vinculin activation is still controversial. However, very recently, additional studies have shed light on how vinculin is activated, in particular at AJ sites.Citation75,Citation76 Choi et al.Citation75 suggest that α-catenin interacts with vinculin by a two-step mechanism in which α-catenin weakly and transiently interacts with the autoinhibited form of vinculin. After unfurling of α-catenin by F-actin, the vinculin binding site of α-catenin becomes more accessible to enable high-affinity binding of the vinculin head region. This event shifts the conformational equilibrium of vinculin from a closed conformation to an open binding state offering the opportunity for vinculin to recruit new actin-binding proteins and consequently to orchestrate the maturation of AJ structures ().
Vinculin is not required for formation of endothelial adherens junctions but instead is necessary for protecting them from opening during the active phase of junction remodeling.Citation77 In fact, recruitment of actin binding proteins by junctional vinculin promotes the reinforcement of connections between the VE-cadherin-catenin complex and the actomyosin filaments leading to the enhancement of cortical actin ring and thus indirectly to the downregulation of endothelial barrier permeability. Additional studies are necessary to show whether the force-dependent recruitment of vinculin and α-actinin at interendothelial AJs operating in static conditions might be also initiated by shear stress.
Interplay between endothelial FAs and AJs
AJs and FAs share several actin-binding proteins such as vinculin,Citation54,Citation71 α-actinin,Citation78 and paxillin.Citation79 In endothelial cell monolayers, we have observed that vinculin and αactinin can shuttle from AJ to FA sites and vice versa.Citation71 In fact, localization of vinculin and α-actinin depends on the tension generated by the actomyosin machinery. For instance, as soon as endothelial cells establish contacts, vinculin and α-actinin progressively accumulate at AJs. Additionally, when the endothelial cell monolayer myosin-II-mediated contractility is blocked by blebbistatin, vinculin and α-actinin translocate from AJs to FAs. An increase in actomyosin tension favors the formation of an AJ-linked cortical actin ring after junctional accumulation of actin-binding proteins to the detriment of actin stress fibers tethered to FAs. Endothelial cells can use this mechanism to modulate the forces exerted at AJs (). We suspect that this behavior is endothelial cell-specific.
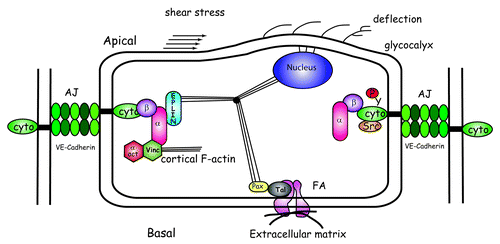
Figure 6. Impact of shear stress on AJ and FA structures. At the apical surface of endothelial cells, shear stress induces the deflection of proteoglycans within the glycocalyx coating leading to deformation of plasma membrane. As endothelial cells elaborate cell–cell contacts, the a-catenin-EPLIN mechanotransmitter recruits vinculin and a-actinin at AJs. This is detrimental for FAs that contain decreasing vinculin and a-actinin. Shear stress activates the kinase Src that tyrosine phosphorylates VE-cadherin.
In addition, processes regulating the actomyosin machinery indirectly participate in mechanotransduction at endothelial AJs. Thus, the Rho A GTPase signaling pathways that regulate the formation of actomyosin filamentsCitation80 and indirectly the cellular contractility may, in turn, control mechanotransduction at AJs. However, there is still some controversy on the role of RhoA in endothelial barrier function. Under static conditions, some studies indicated that inhibiting RhoA function prevents the increase of permeability induced by thrombinCitation81,Citation82 or histamine,Citation81 while others claimed the contrary.Citation83 Nevertheless, under fluid flow conditions, the RhoA pathway seems able to transduce flow-generated forces involved in endothelial barrier function, in particular during the development of cardiac valves in the zebrafish embryo model.Citation84
Mechanotransduction Mechanism Induced by Shear Stress in the Endothelium
In the endothelium, mechanotransduction induced by shear stress requires several successive steps: first, physical deformation of the apical surface of the endothelium; second, intracellular transmission of stress; third, conversion of mechanical forces into biological activity; fourth, downstream intracellular signaling.Citation9
As shear stress acts at the luminal surface of the endothelium, several local structures can initiate mechanotransduction. Recently, much attention has been paid to the glycocalyx, the outer filamentous coating of carbohydrate-rich molecules covering the apical surface of the endothelium. The glycocalyx consists of several types of long proteoglycans anchored to the plasma membrane. Protruding up from the cell surface, they are subjected to shear stress forces and consequently their flexible chains can be deformed under blood flow (). Their deflection probably deforms the plasma membrane thus contributing to force transmission.Citation13 Evidence of the contribution of the glycocalyx to shear stress mechanotransduction comes from studies showing that enzymes able to degrade proteoglycans such as heparinases block responses to shear stress.Citation85
Apical forces generated by shear stress can be transmitted via the cytoskeletal filaments distributed throughout the cell body to distant cellular sites. In endothelial cells, cytoskeletal filaments are interconnected and linked to membrane proteins, in particular to those constituting the adhesive FA and cell–cell junctional structures. Consequently, forces applied at the luminal cell surface can be transmitted to these adhesive structures via cytoskeletal deformations and displacements. In addition, through cell–cell junctions, deformation effects could be propagated to adjoining cells ().
In endothelial cells, shear stress was shown to trigger numerous events such as activation of the kinases ERK (extracellular signal-regulated kinases), JNK (c-jun N-terminal kinases), Src, VEGFR2 (vascular endothelial growth factor-2) and AKT (v-akt murine thymoma viral oncogene homolog) and induction of NF-kB (nuclear factor of kappa light chain gene enhancer in B cells).Citation10 Moreover, a mechanosensory complex expressed at cell-cell junctions and composed of PECAM-1, VE-cadherin and VEGFR2 was shown to transmit a shear-stress signaling pathway from the apical surface of endothelial cells through the cytoskeleton to cell–matrix and cell–cell sites of attachment.Citation86 These data have emerged from experiments performed on endothelial cell lines in which the VE-cadherin or PECAM-1 genes were individually knocked out (VE-cadherin−/− and PECAM-1−/−) prior to be re-expressed (VE-cadherin+/+ and PECAM-1+/+). Shear stress was shown to induce activation of integrins and alignment of actin filaments along flow direction in VEcadherin+/+ and PECAM-1+/+ endothelial cell lines. No such events occur in VE-cadherin-/and PECAM-1−/− endothelial cell lines. This demonstrates the requirement of VE-cadherin and PECAM-1 in the shear stress-induced activation of integrins and downstream events. In addition, initiation of flow mediates VEGFR2 activation in VE-cadherin+/+ and PECAM1+/+ and not in VE-cadherin−/− and PECAM-1−/− endothelial cells indicating that shear stress-induced VEGFR2 activation is downstream of the junctional receptors. In turn, only in cells expressing VE-cadherin, activated VEFGR-2 interacts directly with the PI(3) kinase thus promoting integrin activation. In addition, shear stress mediates the association of PECAM-1 and β-catenin with the PI(3) kinase p85 subunit in a VE-cadherin-dependent manner. Moreover, association between VE-cadherin and VEGFR2 appeared probably indirectly mediated by β-catenin. This correlated with the fact that β-catenin−/− endothelial cells failed to initiate integrin activation under flow. Thus, PECAM-1, VE-cadherin/β-catenin and VEGFR2 represent endothelial-specific mechanotransducer elements required for PI(3)K-mediated integrin activation and endothelial cell alignment with the direction of the flow. In addition, activation of integrins led to activation of NF-kB, an event that promotes atherogenesis under disturbed flow.
Modulation of Endothelial Barrier Function by VE-Cadherin Tyrosine Phosphorylation
It was recently reported that in vivo hemodynamic forces modulate VE-cadherin phosphorylation.Citation87 In fact, VE-cadherin appears phosphorylated on tyrosines Y658 and Y685 in vivo in veins but not in arteries. The difference in the level of shear stress to which endothelial cells are exposed in veins (0.8–8 dynes.cm-2) and in arteries (19–61 dynes.cm-2)Citation88 modulates the activity of the kinase Src at AJs. Junctional Src is activated only in veins and not in arteries, thus explaining the different level of VE-cadherin phosphorylation in veins and arteries.Citation87 Moreover, in vivo phosphorylation of VE-cadherin on tyrosine Y658 and Y685 contributes to the dynamic state of adherens junctions.
There is controversy in the literature about which tyrosine residues of VE-cadherin are phosphorylated after VEGF treatment and about the impact of such phosphorylation events on endothelial barrier function. Some studies reported that VE-cadherin phosphorylation increased the permeability of endothelial monolayers facilitating trans-endothelial migration of leukocytes.Citation89,Citation90 By contrast, other studies claimed that Src-induced tyrosine phosphorylation of VE-cadherin is not sufficient to promote an increase in endothelial cell monolayer permeability.Citation87,Citation91 Further investigations are required to fully understand the mechanisms at the basis of endothelial barrier stabilization, disruption, or remodeling. In particular, it is still unclear whether VE-cadherin tyrosine phosphorylation-mediated signaling and actomyosin machinery-linked mechanotransduction constitute interrelated or parallel pathways. Recently, it was reported that a high degree of tyrosine phosphorylation of VEcadherin, after constitutively active Src overexpression, interferes with the organization of the cytoskeleton in human dermal microvascular endothelial cells.Citation91 In fact a drastic rearrangement of the actin cytoskeleton with formation of thick actin bundles was observed, supporting the notion that both types of signaling are interrelated. Disconnection between VEcadherin and its intracellular partners may explain this strong actin cytoskeleton remodeling.
Tyrosine phosphorylation of VE-cadherin was described to reduce the ability of VEcadherin to interact with its intracellular partners.Citation92 In fact, tyrosine phosphorylation on Y658 and Y731 was reported to be sufficient to prevent binding to the VE-cadherin cytoplasmic part of p120 and β-catenin, respectively, with each tyrosine phosphorylation having the capability to inhibit endothelial barrier function. By contrast, more recent studies have shown that VE-cadherin tyrosine phosphorylation neither modifies the association of VE-cadherin with p120 and β-catenins nor decreases the barrier function of endothelial cell monolayers.Citation87,Citation91 These last data support the notion that tyrosine phosphorylation of VEcadherin is required but is not sufficient to promote the disassembly of adherens junctions. Nevertheless, in our laboratory we have generated knock-in mice carrying within the cytoplasmic part of VE-cadherin the tyrosine-to-phenylalanine point mutation Y685F thus preventing phosphorylation on Y685. These knock-in mice spontaneously exhibited leakier capillaries compared with those of wild-type mice, thus demonstrating that VE-cadherin tyrosine phosphorylation at Y685 participates in the regulation processes of vascular permeability by a still unknown mechanism (unpublished results).
Conclusion
The endothelium undergoes drastic remodeling in pathologies such as pulmonary edema, sepsis, inflammation, vascular leakage, atherosclerosis and tumor-associated angiogenesis. Restoration of endothelial barrier function is critical in resolving these pathological morbid conditions. There is a clear unmet medical need to treat these diseases, which necessitates the discovery of novel therapeutic targets for safe and successful therapies. Further research aimed at investigating the mechanisms at the basis of endothelial barrier function regulation will lead to new treatment approaches for these diseases.
References
- Vollrath MA, Kwan KY, Corey DP. The micromachinery of mechanotransduction in hair cells. Annu Rev Neurosci 2007; 30:339 - 65; http://dx.doi.org/10.1146/annurev.neuro.29.051605.112917; PMID: 17428178
- Hammerschmidt S, Kuhn H, Gessner C, Seyfarth HJ, Wirtz H. Stretch-induced alveolar type II cell apoptosis: role of endogenous bradykinin and PI3K-Akt signaling. Am J Respir Cell Mol Biol 2007; 37:699 - 705; http://dx.doi.org/10.1165/rcmb.2006-0429OC; PMID: 17630321
- Affonce DA, Lutchen KR. New perspectives on the mechanical basis for airway hyperreactivity and airway hypersensitivity in asthma. J Appl Physiol 2006; 101:1710 - 9; http://dx.doi.org/10.1152/japplphysiol.00344.2006; PMID: 16902064
- Heydemann A, McNally EM. Consequences of disrupting the dystrophin-sarcoglycan complex in cardiac and skeletal myopathy. Trends Cardiovasc Med 2007; 17:55 - 9; http://dx.doi.org/10.1016/j.tcm.2006.12.002; PMID: 17292047
- Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol 2009; 10:63 - 73; http://dx.doi.org/10.1038/nrm2597; PMID: 19197333
- Lehoux S, Esposito B, Merval R, Tedgui A. Differential regulation of vascular focal adhesion kinase by steady stretch and pulsatility. Circulation 2005; 111:643 - 9; http://dx.doi.org/10.1161/01.CIR.0000154548.16191.2F; PMID: 15668343
- Lehoux S, Castier Y, Tedgui A. Molecular mechanisms of the vascular responses to haemodynamic forces. J Intern Med 2006; 259:381 - 92; http://dx.doi.org/10.1111/j.1365-2796.2006.01624.x; PMID: 16594906
- Zebda N, Dubrovskyi O, Birukov KG. Focal adhesion kinase regulation of mechanotransduction and its impact on endothelial cell functions. Microvasc Res 2012; 83:71 - 81; http://dx.doi.org/10.1016/j.mvr.2011.06.007; PMID: 21741394
- Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med 2009; 6:16 - 26; http://dx.doi.org/10.1038/ncpcardio1397; PMID: 19029993
- Davies PF. Overview: temporal and spatial relationships in shear stress-mediated endothelial signalling. J Vasc Res 1997; 34:208 - 11; http://dx.doi.org/10.1159/000159224; PMID: 9226302
- Tzima E, del Pozo MA, Shattil SJ, Chien S, Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J 2001; 20:4639 - 47; http://dx.doi.org/10.1093/emboj/20.17.4639; PMID: 11532928
- DeMaio L, Chang YS, Gardner TW, Tarbell JM, Antonetti DA. Shear stress regulates occludin content and phosphorylation. Am J Physiol Heart Circ Physiol 2001; 281:H105 - 13; PMID: 11406474
- Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol 2009; 10:53 - 62; http://dx.doi.org/10.1038/nrm2596; PMID: 19197332
- Tzima E. Role of small GTPases in endothelial cytoskeletal dynamics and the shear stress response. Circ Res 2006; 98:176 - 85; http://dx.doi.org/10.1161/01.RES.0000200162.94463.d7; PMID: 16456110
- Tzima E, Del Pozo MA, Kiosses WB, Mohamed SA, Li S, Chien S, et al. Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. EMBO J 2002; 21:6791 - 800; http://dx.doi.org/10.1093/emboj/cdf688; PMID: 12486000
- Miao H, Hu YL, Shiu YT, Yuan S, Zhao Y, Kaunas R, et al. Effects of flow patterns on the localization and expression of VE-cadherin at vascular endothelial cell junctions: in vivo and in vitro investigations. J Vasc Res 2005; 42:77 - 89; http://dx.doi.org/10.1159/000083094; PMID: 15637443
- Ting LH, Jahn JR, Jung JI, Shuman BR, Feghhi S, Han SJ, et al. Flow mechanotransduction regulates traction forces, intercellular forces and adherens junctions. Am J Physiol Heart Circ Physiol 2012; 302:H2220 - 9; http://dx.doi.org/10.1152/ajpheart.00975.2011; PMID: 22447948
- Colgan OC, Ferguson G, Collins NT, Murphy RP, Meade G, Cahill PA, et al. Regulation of bovine brain microvascular endothelial tight junction assembly and barrier function by laminar shear stress. Am J Physiol Heart Circ Physiol 2007; 292:H3190 - 7; http://dx.doi.org/10.1152/ajpheart.01177.2006; PMID: 17308001
- Phelps JE, DePaola N. Spatial variations in endothelial barrier function in disturbed flows in vitro. Am J Physiol Heart Circ Physiol 2000; 278:H469 - 76; PMID: 10666077
- Liu Z, Tan JL, Cohen DM, Yang MT, Sniadecki NJ, Ruiz SA, et al. Mechanical tugging force regulates the size of cell-cell junctions. Proc Natl Acad Sci U S A 2010; 107:9944 - 9; http://dx.doi.org/10.1073/pnas.0914547107; PMID: 20463286
- Zaidel-Bar R, Geiger B. The switchable integrin adhesome. J Cell Sci 2010; 123:1385 - 8; http://dx.doi.org/10.1242/jcs.066183; PMID: 20410370
- Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, et al. Nanoscale architecture of integrin-based cell adhesions. Nature 2010; 468:580 - 4; http://dx.doi.org/10.1038/nature09621; PMID: 21107430
- Michaelson JE, Huang H. Cell-cell junctional proteins in cardiovascular mechanotransduction. Ann Biomed Eng 2012; 40:568 - 77; http://dx.doi.org/10.1007/s10439-011-0439-6; PMID: 22016325
- Aijaz S, Balda MS, Matter K. Tight junctions: molecular architecture and function. Int Rev Cytol 2006; 248:261 - 98; http://dx.doi.org/10.1016/S0074-7696(06)48005-0; PMID: 16487793
- Luissint AC, Artus C, Glacial F, Ganeshamoorthy K, Couraud PO. Tight junctions at the blood brain barrier: physiological architecture and disease-associated dysregulation. Fluids Barriers CNS 2012; 9:23; http://dx.doi.org/10.1186/2045-8118-9-23; PMID: 23140302
- McCue S, Noria S, Langille BL. Shear-induced reorganization of endothelial cell cytoskeleton and adhesion complexes. Trends Cardiovasc Med 2004; 14:143 - 51; http://dx.doi.org/10.1016/j.tcm.2004.02.003; PMID: 15177265
- de Beco S, Gueudry C, Amblard F, Coscoy S. Endocytosis is required for Ecadherin redistribution at mature adherens junctions. Proc Natl Acad Sci USA 2009; 106:70107015; http://dx.doi.org/10.1073/pnas.0811253106
- Conklin BS, Zhong DS, Zhao W, Lin PH, Chen C. Shear stress regulates occludin and VEGF expression in porcine arterial endothelial cells. J Surg Res 2002; 102:13 - 21; http://dx.doi.org/10.1006/jsre.2001.6295; PMID: 11792146
- Collins NT, Cummins PM, Colgan OC, Ferguson G, Birney YA, Murphy RP, et al. Cyclic strain-mediated regulation of vascular endothelial occludin and ZO-1: influence on intercellular tight junction assembly and function. Arterioscler Thromb Vasc Biol 2006; 26:62 - 8; http://dx.doi.org/10.1161/01.ATV.0000194097.92824.b3; PMID: 16269664
- Tarbell JM. Shear stress and the endothelial transport barrier. Cardiovasc Res 2010; 87:320 - 30; http://dx.doi.org/10.1093/cvr/cvq146; PMID: 20543206
- Nishimura T, Takeichi M. Remodeling of the adherens junctions during morphogenesis. Curr Top Dev Biol 2009; 89:33 - 54; http://dx.doi.org/10.1016/S0070-2153(09)89002-9; PMID: 19737641
- Maruthamuthu V, Aratyn-Schaus Y, Gardel ML. Conserved F-actin dynamics and force transmission at cell adhesions. Curr Opin Cell Biol 2010; 22:583 - 8; http://dx.doi.org/10.1016/j.ceb.2010.07.010; PMID: 20728328
- Chu YS, Thomas WA, Eder O, Pincet F, Perez E, Thiery JP, et al. Force measurements in E-cadherin-mediated cell doublets reveal rapid adhesion strengthened by actin cytoskeleton remodeling through Rac and Cdc42. J Cell Biol 2004; 167:1183 - 94; http://dx.doi.org/10.1083/jcb.200403043; PMID: 15596540
- Chu YS, Eder O, Thomas WA, Simcha I, Pincet F, Ben-Ze’ev A, et al. Prototypical type I E-cadherin and type II cadherin-7 mediate very distinct adhesiveness through their extracellular domains. J Biol Chem 2006; 281:2901 - 10; http://dx.doi.org/10.1074/jbc.M506185200; PMID: 16253998
- le Duc Q, Shi Q, Blonk I, Sonnenberg A, Wang N, Leckband D, et al. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J Cell Biol 2010; 189:1107 - 15; http://dx.doi.org/10.1083/jcb.201001149; PMID: 20584916
- Maruthamuthu V, Sabass B, Schwarz US, Gardel ML. Cell-ECM traction force modulates endogenous tension at cell-cell contacts. Proc Natl Acad Sci U S A 2011; 108:4708 - 13; http://dx.doi.org/10.1073/pnas.1011123108; PMID: 21383129
- Ganz A, Lambert M, Saez A, Silberzan P, Buguin A, Mège RM, et al. Traction forces exerted through N-cadherin contacts. Biol Cell 2006; 98:721 - 30; http://dx.doi.org/10.1042/BC20060039; PMID: 16895521
- Yonemura S. A mechanism of mechanotransduction at the cell-cell interface: emergence of α-catenin as the center of a force-balancing mechanism for morphogenesis in multicellular organisms. Bioessays 2011; 33:732 - 6; http://dx.doi.org/10.1002/bies.201100064; PMID: 21826690
- del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science 2009; 323:638 - 41; http://dx.doi.org/10.1126/science.1162912; PMID: 19179532
- Lee SE, Kamm RD, Mofrad MR. Force-induced activation of talin and its possible role in focal adhesion mechanotransduction. J Biomech 2007; 40:2096 - 106; http://dx.doi.org/10.1016/j.jbiomech.2007.04.006; PMID: 17544431
- Hu K, Ji L, Applegate KT, Danuser G, Waterman-Storer CM. Differential transmission of actin motion within focal adhesions. Science 2007; 315:111 - 5; http://dx.doi.org/10.1126/science.1135085; PMID: 17204653
- Humphries JD, Wang P, Streuli C, Geiger B, Humphries MJ, Ballestrem C. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J Cell Biol 2007; 179:1043 - 57; http://dx.doi.org/10.1083/jcb.200703036; PMID: 18056416
- Liu Z, Tan JL, Cohen DM, Yang MT, Sniadecki NJ, Ruiz SA, et al. Mechanical tugging force regulates the size of cell-cell junctions. Proc Natl Acad Sci U S A 2010; 107:9944 - 9; http://dx.doi.org/10.1073/pnas.0914547107; PMID: 20463286
- Brevier J, Montero D, Svitkina T, Riveline D. The asymmetric self-assembly mechanism of adherens junctions: a cellular push-pull unit. Phys Biol 2008; 5:016005; http://dx.doi.org/10.1088/1478-3975/5/1/016005; PMID: 18379019
- Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol 2004; 5:261 - 70; http://dx.doi.org/10.1038/nrm1357; PMID: 15071551
- Dejana E, Bazzoni G, Lampugnani MG. Vascular endothelial (VE)-cadherin: only an intercellular glue?. Exp Cell Res 1999; 252:13 - 9; http://dx.doi.org/10.1006/excr.1999.4601; PMID: 10502395
- Hordijk PL, Anthony E, Mul FP, Rientsma R, Oomen LC, Roos D. Vascular-endothelial-cadherin modulates endothelial monolayer permeability. [In Process Citation] J Cell Sci 1999; 112:1915 - 23; PMID: 10341210
- Gulino D, Delachanal E, Concord E, Genoux Y, Morand B, Valiron MO, et al. Alteration of endothelial cell monolayer integrity triggers resynthesis of vascular endothelium cadherin. J Biol Chem 1998; 273:29786 - 93; http://dx.doi.org/10.1074/jbc.273.45.29786; PMID: 9792693
- Hirano S, Kimoto N, Shimoyama Y, Hirohashi S, Takeichi M. Identification of a neural alpha-catenin as a key regulator of cadherin function and multicellular organization. Cell 1992; 70:293 - 301; http://dx.doi.org/10.1016/0092-8674(92)90103-J; PMID: 1638632
- Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell 2005; 123:903 - 15; http://dx.doi.org/10.1016/j.cell.2005.09.021; PMID: 16325583
- Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell 2005; 123:889 - 901; http://dx.doi.org/10.1016/j.cell.2005.09.020; PMID: 16325582
- Schulte D, Küppers V, Dartsch N, Broermann A, Li H, Zarbock A, et al. Stabilizing the VE-cadherin-catenin complex blocks leukocyte extravasation and vascular permeability. EMBO J 2011; 30:4157 - 70; http://dx.doi.org/10.1038/emboj.2011.304; PMID: 21857650
- Maiden SL, Hardin J. The secret life of α-catenin: moonlighting in morphogenesis. J Cell Biol 2011; 195:543 - 52; http://dx.doi.org/10.1083/jcb.201103106; PMID: 22084304
- Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol 2010; 12:533 - 42; http://dx.doi.org/10.1038/ncb2055; PMID: 20453849
- Pokutta S, Drees F, Takai Y, Nelson WJ, Weis WI. Biochemical and structural definition of the l-afadin- and actin-binding sites of alpha-catenin. J Biol Chem 2002; 277:18868 - 74; http://dx.doi.org/10.1074/jbc.M201463200; PMID: 11907041
- Nieset JE, Redfield AR, Jin F, Knudsen KA, Johnson KR, Wheelock MJ. Characterization of the interactions of alpha-catenin with alpha-actinin and beta-catenin/plakoglobin. J Cell Sci 1997; 110:1013 - 22; PMID: 9152027
- Kobielak A, Pasolli HA, Fuchs E. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat Cell Biol 2004; 6:21 - 30; http://dx.doi.org/10.1038/ncb1075; PMID: 14647292
- Abe K, Takeichi M. EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt. Proc Natl Acad Sci U S A 2008; 105:13 - 9; http://dx.doi.org/10.1073/pnas.0710504105; PMID: 18093941
- Itoh M, Nagafuchi A, Moroi S, Tsukita S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J Cell Biol 1997; 138:181 - 92; http://dx.doi.org/10.1083/jcb.138.1.181; PMID: 9214391
- Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci 1994; 107:3655 - 63; PMID: 7706414
- Pokutta S, Weis WI. Structure of the dimerization and beta-catenin-binding region of alpha-catenin. Mol Cell 2000; 5:533 - 43; http://dx.doi.org/10.1016/S1097-2765(00)80447-5; PMID: 10882138
- Nagafuchi A, Ishihara S, Tsukita S. The roles of catenins in the cadherin-mediated cell adhesion: functional analysis of E-cadherin-alpha catenin fusion molecules. J Cell Biol 1994; 127:235 - 45; http://dx.doi.org/10.1083/jcb.127.1.235; PMID: 7929566
- Shewan AM, Maddugoda M, Kraemer A, Stehbens SJ, Verma S, Kovacs EM, et al. Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell-cell contacts. Mol Biol Cell 2005; 16:4531 - 42; http://dx.doi.org/10.1091/mbc.E05-04-0330; PMID: 16030252
- Smutny M, Yap AS. Neighborly relations: cadherins and mechanotransduction. J Cell Biol 2010; 189:1075 - 7; http://dx.doi.org/10.1083/jcb.201005151; PMID: 20584914
- Zheng Q, Zhao Y. The diverse biofunctions of LIM domain proteins: determined by subcellular localization and protein-protein interaction. Biol Cell 2007; 99:489 - 502; http://dx.doi.org/10.1042/BC20060126; PMID: 17696879
- Maul RS, Song Y, Amann KJ, Gerbin SC, Pollard TD, Chang DD. EPLIN regulates actin dynamics by cross-linking and stabilizing filaments. J Cell Biol 2003; 160:399 - 407; http://dx.doi.org/10.1083/jcb.200212057; PMID: 12566430
- Jiang WG, Martin TA, Lewis-Russell JM, Douglas-Jones A, Ye L, Mansel RE. Eplin-alpha expression in human breast cancer, the impact on cellular migration and clinical outcome. Mol Cancer 2008; 7:71; http://dx.doi.org/10.1186/1476-4598-7-71; PMID: 18796137
- Han MY, Kosako H, Watanabe T, Hattori S. Extracellular signal-regulated kinase/mitogen-activated protein kinase regulates actin organization and cell motility by phosphorylating the actin cross-linking protein EPLIN. Mol Cell Biol 2007; 27:8190 - 204; http://dx.doi.org/10.1128/MCB.00661-07; PMID: 17875928
- Chircop M, Oakes V, Graham ME, Ma MP, Smith CM, Robinson PJ, et al. The actin-binding and bundling protein, EPLIN, is required for cytokinesis. Cell Cycle 2009; 8:757 - 64; http://dx.doi.org/10.4161/cc.8.5.7878; PMID: 19221476
- Zhang S, Wang X, Iqbal S, Wang Y, Osunkoya AO, Chen Z, et al. Epidermal growth factor promotes protein degradation of epithelial protein lost in neoplasm (EPLIN), a putative metastasis suppressor, during epithelial-mesenchymal transition. J Biol Chem 2012; PMID: 23188829
- Chervin-Pétinot A, Courçon M, Almagro S, Nicolas A, Grichine A, Grunwald D, et al. Epithelial protein lost in neoplasm (EPLIN) interacts with α-catenin and actin filaments in endothelial cells and stabilizes vascular capillary network in vitro. J Biol Chem 2012; 287:7556 - 72; http://dx.doi.org/10.1074/jbc.M111.328682; PMID: 22194609
- Taguchi K, Ishiuchi T, Takeichi M. Mechanosensitive EPLIN-dependent remodeling of adherens junctions regulates epithelial reshaping. J Cell Biol 2011; 194:643 - 56; http://dx.doi.org/10.1083/jcb.201104124; PMID: 21844208
- Ziegler WH, Liddington RC, Critchley DR. The structure and regulation of vinculin. Trends Cell Biol 2006; 16:453 - 60; http://dx.doi.org/10.1016/j.tcb.2006.07.004; PMID: 16893648
- Bois PR, O’Hara BP, Nietlispach D, Kirkpatrick J, Izard T. The vinculin binding sites of talin and alpha-actinin are sufficient to activate vinculin. J Biol Chem 2006; 281:7228 - 36; http://dx.doi.org/10.1074/jbc.M510397200; PMID: 16407299
- Choi HJ, Pokutta S, Cadwell GW, Bobkov AA, Bankston LA, Liddington RC, et al. αE-catenin is an autoinhibited molecule that coactivates vinculin. Proc Natl Acad Sci U S A 2012; 109:8576 - 81; http://dx.doi.org/10.1073/pnas.1203906109; PMID: 22586082
- Peng X, Maiers JL, Choudhury D, Craig SW, DeMali KA. α-Catenin uses a novel mechanism to activate vinculin. J Biol Chem 2012; 287:7728 - 37; http://dx.doi.org/10.1074/jbc.M111.297481; PMID: 22235119
- Huveneers S, Oldenburg J, Spanjaard E, van der Krogt G, Grigoriev I, Akhmanova A, et al. Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. J Cell Biol 2012; 196:641 - 52; http://dx.doi.org/10.1083/jcb.201108120; PMID: 22391038
- Tang VW, Brieher WM. α-Actinin-4/FSGS1 is required for Arp2/3-dependent actin assembly at the adherens junction. J Cell Biol 2012; 196:115 - 30; http://dx.doi.org/10.1083/jcb.201103116; PMID: 22232703
- Sun X, Shikata Y, Wang L, Ohmori K, Watanabe N, Wada J, et al. Enhanced interaction between focal adhesion and adherens junction proteins: involvement in sphingosine 1-phosphate-induced endothelial barrier enhancement. Microvasc Res 2009; 77:304 - 13; http://dx.doi.org/10.1016/j.mvr.2008.12.004; PMID: 19323978
- Olson MF. Contraction reaction: mechanical regulation of Rho GTPase. Trends Cell Biol 2004; 14:111 - 4; http://dx.doi.org/10.1016/j.tcb.2004.01.005; PMID: 15055199
- Wójciak-Stothard B, Potempa S, Eichholtz T, Ridley AJ. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci 2001; 114:1343 - 55; PMID: 11257000
- Carbajal JM, Schaeffer RC Jr.. RhoA inactivation enhances endothelial barrier function. Am J Physiol 1999; 277:C955 - 64; PMID: 10564088
- Vouret-Craviari V, Boquet P, Pouysségur J, Van Obberghen-Schilling E. Regulation of the actin cytoskeleton by thrombin in human endothelial cells: role of Rho proteins in endothelial barrier function. Mol Biol Cell 1998; 9:2639 - 53; PMID: 9725917
- Tan H, Biechler S, Junor L, Yost MJ, Dean D, Li J, et al. Fluid flow forces and rhoA regulate fibrous development of the atrioventricular valves. Dev Biol 2012; PMID: 23261934
- Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng 2007; 9:121 - 67; http://dx.doi.org/10.1146/annurev.bioeng.9.060906.151959; PMID: 17373886
- Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 2005; 437:426 - 31; http://dx.doi.org/10.1038/nature03952; PMID: 16163360
- Orsenigo F, Giampietro C, Ferrari A, Corada M, Galaup A, Sigismund S, et al. Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat Commun 2012; 3:1208; http://dx.doi.org/10.1038/ncomms2199; PMID: 23169049
- Kroll MH, Hellums JD, McIntire LV, Schafer AI, Moake JL. Platelets and shear stress. Blood 1996; 88:1525 - 41; PMID: 8781407
- Turowski P, Martinelli R, Crawford R, Wateridge D, Papageorgiou AP, Lampugnani MG, et al. Phosphorylation of vascular endothelial cadherin controls lymphocyte emigration. J Cell Sci 2008; 121:29 - 37; http://dx.doi.org/10.1242/jcs.022681; PMID: 18096689
- Allingham MJ, van Buul JD, Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol 2007; 179:4053 - 64; PMID: 17785844
- Adam AP, Sharenko AL, Pumiglia K, Vincent PA. Src-induced tyrosine phosphorylation of VE-cadherin is not sufficient to decrease barrier function of endothelial monolayers. J Biol Chem 2010; 285:7045 - 55; http://dx.doi.org/10.1074/jbc.M109.079277; PMID: 20048167
- Potter MD, Barbero S, Cheresh DA. Tyrosine phosphorylation of VE-cadherin prevents binding of p120- and beta-catenin and maintains the cellular mesenchymal state. J Biol Chem 2005; 280:31906 - 12; http://dx.doi.org/10.1074/jbc.M505568200; PMID: 16027153
- Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol 2010; 11:502 - 14; http://dx.doi.org/10.1038/nrm2927; PMID: 20571587