Abstract
Focal adhesion kinase (FAK), as its name implied, is an important mediator of integrin-based signaling function in mammalian cells at the focal adhesion complex (FAC, also known as focal contact) at the cell-extracellular matrix interface. FAK is intimately related to cell movement, such as in macrophages, fibroblasts and also tumor cells. In the testis, however, FAK and two of its phosphorylated forms, p-FAK-Tyr407 and -Tyr397, are not found at the FAC since there is no ultrastructure analogous or similar to FAC in the mammalian testis vs. other epithelia. Instead, FAK and its two phosphorylated forms are detected along the seminiferous epithelium in the rat testis at the cell-cell interface in a testis-specific adherens junction (AJ) known as the ectoplasmic specialization (ES). ES is an F-actin-rich ultrastructure in which bundles of actin filaments are sandwiched in-between plasma membrane and cisternae of endoplasmic reticulum not found in other mammalian epithelial/endothelial cells. The ES is restricted to the interface of Sertoli cells and spermatids (step 8–19) known as the apical ES, and to the Sertoli cell-cell interface known as the basal ES. Interestingly, the basal ES is also an integrated component of the blood-testis barrier (BTB), coexisting with tight junction (TJ) and gap junction (GJ), and it is conceivable that actin filament bundles at the ES undergo extensive organization, converting from their “bundled” to “de-bundled/branching” configuration to facilitate transport of germ cells across the epithelium and at the BTB during the epithelial cycle. A recent report (Lie et al. PNAS 109:12562–12567, 2012) has demonstrated that the stage-specific and spatiotemporal expression of p-FAK-Tyr407 and -Tyr397 are crucial to the regulation of these events via their stage-specific and spatiotemporal expression during the epithelial cycle mediated by their effects on the organization of the actin filament bundles at the ES, involving actin binding/regulatory proteins. In this Commentary, we will critically evaluate these findings in light of other recent reports in the field. While these ideas are based on studies in the BTB in the rat testis, this information should be applicable and helpful to investigators studying other tissue barriers.
Introduction
The seminiferous tubule is the functional unit in the mammalian testis, such as in human males, which produces millions of spermatozoa each day since puberty.Citation1-Citation3 This is made possible since spermatogonial stem cells (SSC) found in the SSC niche,Citation4,Citation5 such as in rodents, are capable of self-renewal and give rise to type A spermatogonia. Each of the type A spermatogonia known as Asingle (or As), being considered as a SSC,Citation4 undergoes a series of mitotic divisions while differentiating into more advanced germ cells, to be followed by meiosis to give rise to 4096 spermatids, which, theoretically can transform into spermatozoa,Citation5,Citation6 and each spermatozoan is capable of fertilizing an egg. Even though many of these germ cells enter apoptosis to avoid overwhelming the fixed number of Sertoli cells in the testis, such that a Sertoli:germ cell ratio of ~1:30–1:50Citation7,Citation8 can be maintained, there are virtually unlimited supplies of sperm cells in the mammalian testis throughout the entire adulthood due to the self-renewal capability of SSC to give rise to spermatogonia.
In the cross-section of a seminiferous tubule, SSC, spermatogonia (different type A spermatogonia, as well as intermediate and type B spermatogonia), and preleptotene spermatocytes differentiated from type B spermatogonia, are all located in the basal compartment, outside the blood-testis barrier (BTB), created by tight junction (TJ), basal ectoplasmic specialization [ES, a testis-specific adherens junction (AJ)], gap junction (GJ) as well as desmosome between adjacent Sertoli cells near the basement membrane (). Preleptotene spermatocytes, however, are the only germ cells that can cross the BTB that take place at stage VIII of the epithelial cycle while transforming and differentiating into leptotene and zygotene spermatocytes, so that late pachytene spermatocytes can undergo diakinesis to prepare for meiosis I/II behind the BTB in the specialized microenvironment in the seminiferous epithelium known as the adluminal compartment (). In short, meiosis I/II and all the post-meiotic spermatid development are segregated from the systemic circulation due to the BTB, and spermatid development during spermiogenesis rely exclusively on the structural and nourishment supports of the Sertoli cell in the adluminal compartment ().
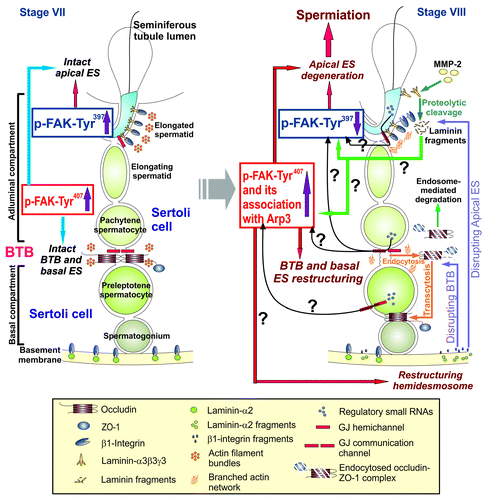
Figure 1. A schematic drawing of a hypothetic model depicting the likely role of two FAK phosphorylated forms, p-FAK-Tyr397 and p-FAK-Tyr407, in conferring plasticity to the actin filament bundles at the ES via their stage-specific and spatiotemporal expression in the seminiferous epithelium during the epithelial cycle of spermatogenesis. On the left panel, it is a schematic drawing of the seminiferous epithelium of a stage VII tubule wherein intact filament bundles at the apical ES and the basal ES are maintained by an upregulation on the expression of p-FAK-Tyr397 and p-FAK-Tyr407, respectively, via the intrinsic activity of Eps8 and other actin filament bundling proteins (e.g., palladin) to confer ES adhesion function at both sites. However, in stage VIII tubules (see right panel), downregulation of p-FAK-Tyr397 but changes in the association of p-FAK-Tyr407 with Arp3 (or with other actin bundling proteins)―even though the expression of p-FAK-Tyr407 remains upregulated at this stage―favor re-organization of the actin filament bundles (such as from their “bundled” to their “un-bundled/branched” configuration in the microdomain within the BTB (see text for details) to induce BTB restructuring at the basal ES to accommodate the transport of preleptotene spermatocytes across the BTB and spermiation at the apical ES. Many questions (annotated by “?”) remain unanswered as noted in this hypothetical model. For instance, it is not known if biologically active fragments released at the apical ES during spermiation or at the hemidesmosome can regulate the spatiotemporal expression of p-FAK-Tyr397 and p-FAK-Tyr407, and/or the interaction between p-FAK-Tyr407 and actin regulatory proteins (e.g., Arp3) to recruit these proteins to the site to induce actin filament reorganization. Also, it remains to be determined if small RNAs that are abundantly found in germ cells are being used to regulate the spatiotemporal expression of these two activated FAK forms in the apical ES-BTB-basement membrane functional axis. Also, it remains to be determined if these small regulatory RNAs are being transported from germ cells to Sertoli cells via gap junctions at different stages of the epithelial cycle.
Interestingly, using electron microscopy, extensively bundles of actin filaments are noted at the Sertoli cell-cell interface at the BTB, coexisting with either TJs or GJs, and this ultrastructure is known as the basal ES (). Similar network of actin filament bundles are also found at the Sertoli cell-spermatid interface (from step 8–19 spermatids in the rat testis) known as the apical ES, except that once the apical ES appears, it is the only anchorage device, replacing GJs and desmosomes. Unlike the basal ES, the apical ES never coexists with other junctions (). This unusual network of actin filament bundles confers strong adhesive function to the BTB, making it one of the tightest blood-tissue barriers in mammals;Citation6,Citation9-Citation11 and it also confers strong adhesion to developing spermatids, making the apical ES one of the strongest anchoring junctions, almost twice as strong as the desmosome when the “force” required to tear the apical ES apart vs. the desmosome was quantified.Citation12
Interestingly, the apical and the basal ES undergo extensive restructuring during the epithelial cycle of spermatogenesis since spermatids are transported by the Sertoli cell across the seminiferous epithelium during the epithelial cycle, whereas preleptotene spermatocytes are also transported across the BTB at stage VIII of the epithelial cycle. Thus, it is envisioned that these actin filament bundles must be re-organized continuously throughout the epithelial cycle, converting from their “bundled” to a “de-bundled” configuration, such that these strong adhesion junction can be “opened/disassembled” to facilitate the transport of spermatids and preleptotene spermatocytes across the epithelium and the BTB, respectively.
Proteins That Regulate the Organization of Actin Filament Bundles at the ES
Eps8 and the Arp2/3 complex
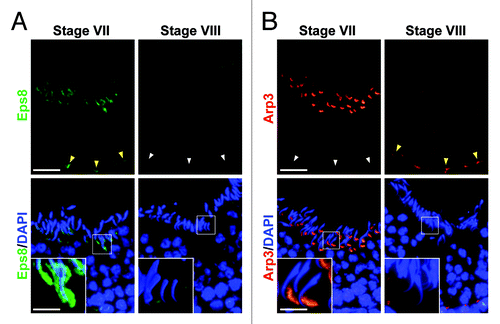
Eps8 (epidermal growth factor receptor pathway substrate 8), an actin barbed end capping and bundling protein,Citation13,Citation14 was shown to confer actin filament bundles at the ES in the rat testis, maintaining the ES integrity during the epithelial cycle via its stage-specific spatiotemporal expression at the apical and basal ES.Citation15 Arp2/3 (actin related protein 2/3) together with ARPC1–5 (actin related protein 2/3 complex subunits 1 to 5) form a 7 subunit Arp2/3 complex. When the Arp2/3 complex is activated by N-WASP (neuronal Wiskott-Aldrich syndrome protein), it induces barbed end nucleation in existing actin filaments complex, inducing branched actin polymerization that converts “bundled” actin filaments to a “de-bundled” configuration. This thus replaces bundled actin filaments with a branched actin network. A recent study has shown that the Arp2/3 complex that induces re-organization of actin filament bundles at the ES, also displays a its stage-specific spatiotemporal expression at the apical and basal ES.Citation16 In short, the antagonistic action of these two proteins at the ES via their spatiotemporal and stage-specific expression can efficiently confer actin filaments their “bundled” and “de-bundled” configuration. Data published in recent years regarding the stage-specific and spatiotemporal expression of Eps8Citation15 and Arp3Citation16 are now summarized in , which clearly illustrate the important concept that the differential spatiotemporal expression of these two proteins at the ES is crucial to the maintenance of actin filament bundles at the apical and the basal ES sites. For instance, in stage VII tubules when step 19 spermatids remain attached to the epithelium with intact apical ES, Eps8 expression is considerably high at the apical ES, surrounding the entire head of the step 19 spermatids; and it is also high at the basal ES when BTB also remains intact (see yellow arrowhead) (). Arp3 expression is also considerably high in stage VII tubules at the apical ES, however, it is restricted to the concave side of the step19 spermatid head, which is the site where extensive endocytic vesicle-mediated protein trafficking takes placeCitation17,Citation18 so that “old” apical ES adhesion protein complexes (e.g., laminin-α3β3γ3, α6β1-integrin, nectins) can be internalized (mediated by a “disruption” of the apical ES at the concave side of the spermatid head due to the formation of a branched actin network), transcytosed and recycled to assemble “newly” apical ES from step 8 spermatids at stage VIII of the cycle via spermiogenesis as they are being transported across the epithelium (). Yet, step 19 spermatids are still attached to the Sertoli cell in the epithelium at stage VII because Eps8 are surrounding the entire head of these spermatids, such as at the convex side ( vs. 2A). It is noted that Eps8 and Arp3 were partially co-localized at the apical ES, such as at stage VII of the epithelial cycle.Citation15,Citation16 However, it is also noted that in stage VII tubules, virtually no Arp3 is detected at the BTB (see white arrowheads in ). In stage VIII tubules, when spermiation and BTB restructuring take place simultaneously, the expression of Eps8 at the apical and the basal ES is virtually non-detectable so that actin filament bundles can be converted to a “de-bundled” state to facilitate these events (). However, Apr3 is also not needed at the apical ES because of the release of the sperm at spermiation in stage VIII tubules. But the expression of Arp3 is high at the basal ES to facilitate BTB restructuring while the expression of Eps8 at the basal ES is virtually non-detectable ( vs. 2A). These findings illustrate an efficient physiological mechanism is in place in the testis to regulate the dynamic organization of actin filaments at the ES during the epithelial cycle from their “bundled” to their “debundled/branched” configuration via the intrinsic, but antagonistic, activities of two actin regulatory proteins. In short, Eps8 favors but Arp3 promotes “bundled” and “debundled/branched” actin configuration, respectively, at the ES during the epithelial cycle of spermatogenesis .
Figure 2. Stage-specific/spatiotemporal expression of Eps8 and Arp3 in the rat testis. (A) At stage VII, Eps8 (green fluorescence), an actin barbed end capping and bundling protein, highly expressed at the spermatid head (apical ES) to confer adhesion, and also at the basal ES to confer BTB integrity (“yellow” arrowheads) in the seminiferous epithelium via its intrinsic activity to maintain the integrity of the actin filament bundles at the ES. At stage VIII, Eps8 expression was almost undetectable: (1) at the apical ES to facilitate spermiation, and (2) at the basal ES (“white” arrowheads) to facilitate BTB restructuring to allow the crossing of preleptotene spermatocytes that allows actin filaments to be converted from their “bundled” to their “de-bundled/branched” configuration mediated by the Arp2/3 complex. (B) Arp3 (red fluorescence), known to induce branched actin polymerization, converting actin bundles to a branched state, its expression was virtually not detected at the basal ES when BTB was intact at stage VII (see “white” arrowheads) of the cycle, but its expression was high at the concave side of the spermatid head via its intrinsic activity that converts actin filaments from their “bundled” to their “de-bundled/branched” configuration, destabilizing the actin cytoskeleton at the site, where endocytic vesicle-mediated protein trafficking is known to take place, so that “old” apical ES proteins are recycled to assemble “new” apical ES ; and Eps8 at the convex side of spermatid head continues to maintain spermatid adhesion via its intrinsic activity to confer actin fialments into their “bundled” configuration. At stage VIII, the expression of Arp3 was diminished and not detectable at the apical ES due to spermiation but Arp3 expression was high at the basal ES to induce BTB restructuring (“yellow” arrowheads). In short, utilizing the stage-specific and spatiotemporal expression of these two actin regulatory proteins that have antagonistic effects on the configuration of actin filament bundles at the ES, they confer “plasticity” to the apical and the basal ES during the epithelial cycle of spermatogenesis to accommodate changes in the adhesion function at the Sertoli-spermatid and Sertoli-Sertoli cell-cell interface to facilitate germ cell adhesion/transport in the epithelium. Bar in (A) and (B), 50 μm; inset in (A) and (B), 15 μm, the magnified view of the corresponding boxed area; which apply to remaining micrographs in both panels.
Drebrin E and the Scribble/Lgl/Dlg complex
Recent studies have shown that the antagonistic actions of these two proteins are being assisted by other proteins at these sites, such as drebrin E, which was shown to have high affinity with Arp3, but not Eps8, and displaying similar spatiotemporal and stage-specific expression as of Apr3 in the seminiferous epithelium of rat testes.Citation19 Furthermore, recent studies have shown that the F-actin network at the ES is also maintained and regulated by the Scribble/Lgl/Dlg polarity proteins.Citation20 This conclusion is based on the observations that the simultaneous knockdown of Scribble and its two partner component proteins Lgl2 and Dlg1 in the testis in vivo was found to induce defects in spermatid polarity since adhesion proteins (e.g., laminin-γ3) were mis-localized, failing to confer proper spermatid polarity in stage VII-VIII tubules; and occludin was also failed to undergo proper re-distribution in stage VIII tubules by moving away from the BTB to facilitate restructuring of the barrier to accommodate the transit of preleptotene spermatocytes at the site, its “unwanted” presence in stage VIII tubules thereby strengthening the Sertoli cell TJ-permeability barrier.Citation20 In short, the proper functioning of the ES in the epithelium during the epithelial cycle requires the stage-specific, spatiotemporal and also differential expression of Eps8 and Arp3, coupled with other supporting proteins (e.g., drebrin E), as well as other regulatory proteins [e.g., the Scribble/Dlg/Lgl complex, secreted Frizzled-related protein 1 (sFRP1Citation21)]. More important, the intrinsic activities of these proteins, at least the Arp2/3 complex, appear to be regulated by the differential expression of a crucial signaling molecule FAK at the ES.Citation22
FAK and Its Two Phosphorylation Forms: p-FAK-Tyr407 and p-FAK-Tyr397
Earlier studies have demonstrated that there is a local functional axis designated the apical ES-BTB-basement membraneCitation23,Citation24 that coordinates cellular events that take place across the seminiferous epithelium during the epithelial cycle, such as the events of spermiation and BTB restructuring which occur simultaneously at the opposite ends of the seminiferous epithelium at stage VIII of the cycle.Citation6,Citation25,Citation26 This functional axis and its physiological significance have recently been confirmed by others using the phthalate-induced Sertoli cell injury modelCitation27-Citation29 and reviewed.Citation30-Citation32 Studies have shown that the subtle changes detected at these sites during these cellular events, such as the release of sperm at spermiation and BTB restructuring that accommodate the transit of preleptotene spermatocytes across the BTB, are largely mediated by changes in the organization of the actin filament bundles, which in turn induce changes in cell adhesion functions at the apical and the basal ES.Citation20,Citation33 While the findings summarized above suggest that re-organization of the actin filament bundles at the apical and the basal ES during the epithelial cycle is regulated by Eps8 and the Arp2/3 complex plus several partner proteins (e.g., drebrin E, the Scribble/Dlg/Lgl, sFRP1), the underlying signaling molecules remain unknown. A recent report from our laboratory has shown that the two phosphorylated forms of FAK, p-FAK-Tyr407 and -Tyr397, are the likely regulatory molecules in this axis that mediate their effects on the F-actin organization at the ES.Citation22 It was shown that p-FAK-Tyr407 and -Tyr307 displayed stage-specific and spatiotemporal expression at the ES in which p-FAK-Tyr407 highly expressed and localized to the apical ES (restricted mostly to the concave side of spermatid heads where endocyic vesicle-mediated protein trafficking take place) and the basal ES at the BTB in stage VII-VIII tubules, likely being used to facilitate ES restructuring.Citation22 This concept is supported by the finding that p-FAK-Tyr407 structurally interacts with Arp3 and N-WASP, but not Esp8, and such interaction increases by at least 3-fold following overexpression of a phosphomimetic mutant of FAK-Y407E.Citation22 However, p-FAK-Tyr397 is also highly expressed at the apical ES but restricted almost exclusively to the convex side of spermatid heads and co-localized with β1-integrin (a putative apical ES protein), conferring spermatid adhesion in stage VII-VIII tubules until spermiation takes place at late stage VIII, but not detectable at the basal ES.Citation22 In short, p-FAK-Tyr397 appears to be important to confer and promote the apical ES adhesion function, but it appears to have an antagonistic effect to the basal ES function. This possibility is also supported by the finding that overexpression of a non-phosphorylatable mutant FAK-Y397F promotes the Sertoli cell tight junction (TJ)-permeability barrier function, making the TJ barrier tighter.Citation22 In short, these findings illustrate that p-FAK-Tyr407 promotes Sertoli cell BTB integrity at the basal ES and endocytic vesicle-mediated protein trafficking at the apical ES, whereas p-FAK-Tyr397 promotes apical ES adhesion and promotes basal ES disruption that perturbs the BTB integrity. However, it is striking to observe that the promoting effects of p-FAK-Tyr407 on the Sertoli cell BTB function is mediated via its association with Arp3 and an increase in actin polymerization rate,Citation22 suggesting that while Arp3 promotes branched actin polymerization which favors the conversion of actin filaments from their “bundled” to their “debundled” and “branched” configuration, but if this intrinsic activity is confined to a specific cellular microdomain within the Sertoli cell BTB microenvironment, such as in the Sertoli cell cytosol instead of at the cell-cell interface, a functional and intact TJ-permeability barrier can still be maintained. Indeed, it was shown that when FAK Y407E was overexpressed in Sertoli cells and there was a ~3-fold increase in association between FAK Y407E and the N-WASP/Arp3 complex, actin filament bundles in the Sertoli cell cytosol were found to be re-organized and re-distributed in which actin filament bundles were localized mostly at the Sertoli cell-cell interface instead of spreading across the Sertoli cell cytosol as seen in the control cells.Citation22 Thus, it is likely that such an increase in FAK Y407E expression activates the Arp2/3-N-WASP complex to induce re-organization of the F-actin at the Sertoli cell BTB in which these bundles of actin filaments are re-organized and re-distributed to the Sertoli cell-cell interface that promotes the integrity of the Sertoli cell TJ-permeability barrier instead of evenly distributed across the cell cytosol. If this concept is true, it is likely that an actin cross-linking and bundling protein in the testis, such as palladin,Citation34 is involved in this event since Eps8 was shown not to be structurally associated/interacted with p-FAK-Tyr407.Citation22 In short, we speculate that palladin may be working in coordination with the Arp2/3-N-WASP complex in which actin filament bundles are “de-bundled” across the cell cytosol via the action of the Arp2/3-N-WASP complex, whereas new actin filament bundles are actively formed at the Sertoli cell-cell interface via the action of palladin. This speculation is supported by a recent report illustrating that palladin exhibits highly stage-specific and spatiotemporal expression at the ES in the rat testis, and its knockdown by RNAi was also found to perturb both the apical ES and the basal ES function, such as the loss of spermatid polarity and Sertoli cell TJ-barrier function, respectively.Citation35 Nonetheless, effects of overexpression these FAK mutants on the distribution of palladin and/or association with a specific FAK mutant in Sertoli cells remain to be established. Furthermore, filamin A, a cross-linker of actin filaments in mammalian cells including Sertoli cells,Citation36 was shown to be crucial to recruiting adhesion protein complexes (e.g., JAM-A/ZO-1, N-cadherin/β-catenin) to the BTB for its assembly during postnatal development in the rat testis,Citation37 may also be involved in the BTB homeostasis mediated by p-FAK-Tyr407 and -Tyr397. Nonetheless, these findings illustrate that much work is needed to better understand the intriguing functional and physiological interactions between FAK and its phosphorylated forms (e.g., p-FAK-Tyr407, p-FAK-Tyr397), actin regulatory proteins (e.g., Eps8, the Arp2/3 complex, palladin, filamin A) and polarity proteins (e.g., the Scribble/Lgl/Dlg complex, Par6, Cdc42). It is obvious that some other yet-to-be identified proteins may also take part in these events.
Concluding Remarks and Future Perspectives
As briefly reviewed herein, FAK, in particular its two phosphorylated forms, p-FAK-Tyr407 and -Tyr397, are important regulators of ES dynamics in the seminiferous epithelium of the rat testis during the epithelial cycle via their effects on the network of actin filament bundles at the apical and the basal ES, which are mediated via their effects on actin regulatory proteins, such as the Arp2/3 complex. They thus affect spermatid adhesion at the apical ES and also Sertoli cell adhesion at the BTB, thereby coordinating cellular events of spermiation and BTB restructuring that take place simultaneously at opposite ends of the epithelium during the epithelial cycle, such as in stage VIII tubules (), at the apical ES-BTB-basement membrane axis. However, there are many questions remain unanswered as illustrated in . For instance, it is likely that other actin cross-linker and/or bundling proteins also take part in the events mediated by these FAK phosphorylated forms, such as filamin A and palladin, as Eps8 was shown not be a participating molecule.Citation22 Furthermore, recent studies have shown that mammalian target of rapamycin complex 1 (mTORC1) and mTORC2;Citation38-Citation40 as well as intercellular adhesion molecule-1 (ICAM-1) and its soluble form (sICAM-1)Citation41 are also having antagonistic effects on the Sertoli cell adhesion function in the testis. Furthermore, it remains to be determined the regulatory molecules that modulate the stage-specific and spatiotemporal expression of p-FAK-Tyr397 and -Tyr407 at the ES. For instance, are these changes mediated by the biologically active fragments of laminins released at the apical ES and/or at the hemidesmosome? Can it be possible that small regulatory RNAs (e.g., miRNA) found in germ cellsCitation42-Citation44 modulate the spatiotemporal expression of these FAK phosphorylated forms via the GJ communication channels?Citation45,Citation46 Many of these questions remain to be addressed in future studies. In short, it will be of interest to investigate the physiological and molecular relationship between these molecules in regulating BTB function at the apical ES-BTB-basement membrane axis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (NICHD, R01 HD056034 to CYC, U54 HD029990 Project 5 to CYC).
References
- Steinberger E. Hormonal control of mammalian spermatogenesis. Physiol Rev 1971; 51:1 - 22
- Sharpe RM. Regulation of spermatogenesis. In: The Physiology of Reproduction. Eds. Knobil, E., Neill, J.D. New York, Raven Press. pp. 1363-1434 (1994).
- O'Donnell L, Meachem SJ, Stanton PG, McLachlan RI. Endocrine regulation of spermatogenesis. In: Neill JD Ed. Physiology of Reproduction, 3rd Edition. Amsterdam, Elsevier. pp. 1017-1069. (2006).
- de Rooij DG. The spermatogonial stem cell niche. Microsc Res Tech 2009; 72:580 - 5; http://dx.doi.org/10.1002/jemt.20699; PMID: 19263493
- Ehmcke J, Wistuba J, Schlatt S. Spermatogonial stem cells: questions, models and perspectives. Hum Reprod Update 2006; 12:275 - 82; http://dx.doi.org/10.1093/humupd/dmk001; PMID: 16446319
- Cheng CY, Mruk DD. The blood-testis barrier and its implications for male contraception. Pharmacol Rev 2012; 64:16 - 64; http://dx.doi.org/10.1124/pr.110.002790; PMID: 22039149
- Wong V, Russell LD. Three-dimensional reconstruction of a rat stage V Sertoli cell: I. Methods, basic configuration, and dimensions. Am J Anat 1983; 167:143 - 61; http://dx.doi.org/10.1002/aja.1001670202; PMID: 6351582
- Russell LD, Tallon-Doran M, Weber JE, Wong V, Peterson RN. Three-dimensional reconstruction of a rat stage V Sertoli cell: III. A study of specific cellular relationships. Am J Anat 1983; 167:181 - 92; http://dx.doi.org/10.1002/aja.1001670204; PMID: 6613903
- Setchell BP. Blood-testis barrier, junctional and transport proteins and spermatogenesis. Adv Exp Med Biol 2008; 636:212 - 33; http://dx.doi.org/10.1007/978-0-387-09597-4_12; PMID: 19856170
- Setchell BP, Waites GMB. The blood-testis barrier. in The Handbook of Physiology. Section 7, Vol. V. Male Reproductive System (eds. Hamilton, D.W. & Greep, R.O.) 143-172 (American Physiological Society, Washington, D.C., 1975).
- Pelletier RM. The blood-testis barrier: the junctional permeability, the proteins and the lipids. Prog Histochem Cytochem 2011; 46:49 - 127; http://dx.doi.org/10.1016/j.proghi.2011.05.001; PMID: 21705043
- Wolski KM, Perrault C, Tran-Son-Tay R, Cameron DF. Strength measurement of the Sertoli-spermatid junctional complex. J Androl 2005; 26:354 - 9; http://dx.doi.org/10.2164/jandrol.04142; PMID: 15867003
- Di Fiore PP, Scita G. Eps8 in the midst of GTPases. Int J Biochem Cell Biol 2002; 34:1178 - 83; http://dx.doi.org/10.1016/S1357-2725(02)00064-X; PMID: 12127568
- Cheng CY, Mruk DD. Regulation of spermiogenesis, spermiation and blood-testis barrier dynamics: novel insights from studies on Eps8 and Arp3. Biochem J 2011; 435:553 - 62; http://dx.doi.org/10.1042/BJ20102121; PMID: 21486226
- Lie PPY, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J 2009; 23:2555 - 67; http://dx.doi.org/10.1096/fj.06-070573; PMID: 19293393
- Lie PPY, Chan AYN, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci U S A 2010; 107:11411 - 6; http://dx.doi.org/10.1073/pnas.1001823107; PMID: 20534520
- Young JS, Takai YK, Kojic KL, Vogl AW. Internalization of adhesion junction proteins and their association with recycling endosome marker proteins in rat seminiferous epithelium. Reproduction 2012; 143:347 - 57; http://dx.doi.org/10.1530/REP-11-0317; PMID: 22157319
- Young JS, Guttman JA, Vaid KS, Vogl AW. Tubulobulbar complexes are intercellular podosome-like structures that internalize intact intercellular junctions during epithelial remodeling events in the rat testis. Biol Reprod 2009; 80:162 - 74; http://dx.doi.org/10.1095/biolreprod.108.070623; PMID: 18799754
- Li MWM, Xiao X, Mruk DD, Lam YL, Lee WM, Lui WY, et al. Actin-binding protein drebrin E is involved in junction dynamics during spermatogenesis. Spermatogenesis 2011; 1:123 - 36; http://dx.doi.org/10.4161/spmg.1.2.16393; PMID: 22319661
- Su WH, Wong EWP, Mruk DD, Cheng CY. The Scribble/Lgl/Dlg polarity protein complex is a regulator of blood-testis barrier dynamics and spermatid polarity during spermatogenesis. Endocrinology 2012; 153:6041 - 53; http://dx.doi.org/10.1210/en.2012-1670; PMID: 23038739
- Wong EWP, Lee WM, Cheng CY. Secreted Frizzled-related protein 1 (sFRP1) regulates spermatid adhesion in the testis via dephosphorylation of focal adhesion kinase and the nectin-3 adhesion protein complex. FASEB J 2013; 27:464 - 77; http://dx.doi.org/10.1096/fj.12-212514; PMID: 23073828 DOI:) (2013).
- Lie PPY, Mruk DD, Mok KW, Su L, Lee WM, Cheng CY. Focal adhesion kinase-Tyr407 and -Tyr397 exhibit antagonistic effects on blood-testis barrier dynamics in the rat. Proc Natl Acad Sci U S A 2012; 109:12562 - 7; http://dx.doi.org/10.1073/pnas.1202316109; PMID: 22797892
- Yan HHN, Mruk DD, Wong EWP, Lee WM, Cheng CY. An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci U S A 2008; 105:8950 - 5; http://dx.doi.org/10.1073/pnas.0711264105; PMID: 18579774
- Wong EWP, Cheng CY. NC1 domain of collagen α3(IV) derived from the basement membrane regulates Sertoli cell blood-testis barrier dynamics. Reproduction 2013; submitted
- Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: role in contraceptive development. Pharmacol Rev 2008; 60:146 - 80; http://dx.doi.org/10.1124/pr.107.07105; PMID: 18483144
- Wong EWP, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochim Biophys Acta 2008; 1778:692 - 708; http://dx.doi.org/10.1016/j.bbamem.2007.11.006; PMID: 18068662
- Yao PL, Lin YC, Richburg JH. TNF α-mediated disruption of spermatogenesis in response to Sertoli cell injury in rodents is partially regulated by MMP2. Biol Reprod 2009; 80:581 - 9; http://dx.doi.org/10.1095/biolreprod.108.073122; PMID: 19038859
- Yao PL, Lin YC, Richburg JH. Mono-(2-ethylhexyl) phthalate-induced disruption of junctional complexes in the seminiferous epithelium of the rodent testis is mediated by MMP2. Biol Reprod 2010; 82:516 - 27; http://dx.doi.org/10.1095/biolreprod.109.080374; PMID: 19828778
- Yao PL, Lin YC, Richburg JH. Transcriptional suppression of Sertoli cell Timp2 in rodents following mono-(2-ethylhexyl) phthalate exposure is regulated by CEBPA and MYC. Biol Reprod 2011; 85:1203 - 15; http://dx.doi.org/10.1095/biolreprod.111.093484; PMID: 21832167
- Mazaud-Guittot S. Dissecting the phthalate-induced Sertoli cell injury: the fragile balance of proteases and their inhibitors. Biol Reprod 2011; 85:1091 - 3; http://dx.doi.org/10.1095/biolreprod.111.095976; PMID: 21900678
- Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nat Rev Endocrinol 2010; 6:380 - 95; http://dx.doi.org/10.1038/nrendo.2010.71; PMID: 20571538
- Cheng CY, Mruk DD. An intracellular trafficking pathway in the seminiferous epithelium regulating spermatogenesis: a biochemical and molecular perspective. Crit Rev Biochem Mol Biol 2009; 44:245 - 63; http://dx.doi.org/10.1080/10409230903061207; PMID: 19622063
- Xiao X, Mruk DD, Cheng CY. c-Yes regulates cell adhesion at the apical ectoplasmic specialization-blood-testis barrier axis via its effects on protein recruitment and distribution. Am J Physiol Endocrinol Metab 2013; 304:E145 - 59; http://dx.doi.org/10.1152/ajpendo.00422.2012; PMID: 23169788
- Qian X, Mruk DD, Cheng YH, Cheng CY. Actin cross-linking protein palladin and spermatogenesis. Spermatogenesis 2013; 3:e23473
- Qian X, Mruk DD, Wong EWP, Lie PPY, Cheng CY. Palladin is a regulator of actin filament bundles at the ectoplasmic specialization in the rat testis. Endocrinology 2013; In press http://dx.doi.org/10.1210/en.2012-2269
- Su WH, Mruk DD, Cheng CY. Filamin A: A regulator of blood-testis barrier assembly during post-natal development. Spermatogenesis 2012; 2:73 - 8; http://dx.doi.org/10.4161/spmg.20223; PMID: 22670216
- Su WH, Mruk DD, Lie PPY, Lui WY, Cheng CY. Filamin A is a regulator of blood-testis barrier assembly during postnatal development in the rat testis. Endocrinology 2012; 153:5023 - 35; http://dx.doi.org/10.1210/en.2012-1286; PMID: 22872576
- Mok KW, Mruk DD, Silvestrini B, Cheng CY. rpS6 Regulates blood-testis barrier dynamics by affecting F-actin organization and protein recruitment. Endocrinology 2012; 153:5036 - 48; http://dx.doi.org/10.1210/en.2012-1665; PMID: 22948214
- Mok KW, Mruk DD, Lee WM, Cheng CY. Rictor/mTORC2 regulates blood-testis barrier dynamics via its effects on gap junction communications and actin filament network. FASEB J 2013; 27:1137 - 52; http://dx.doi.org/10.1096/fj.12-212977; PMID: 23288930
- Mok KW, Mruk DD, Cheng CY. Regulation of blood-testis barrier (BTB) dynamics during spermatogenesis via the “Yin” and “Yang” effects of mammalian target of rapamycin complex 1 (mTORC1) and mTORC2. Int Rev Cell Mol Biol 2013; 301:291 - 358; http://dx.doi.org/10.1016/B978-0-12-407704-1.00006-3; PMID: 23317821
- Xiao X, Cheng CY, Mruk DD. Intercellular adhesion molecule-1 is a regulator of blood-testis barrier function. J Cell Sci 2012; 125:5677 - 89; http://dx.doi.org/10.1242/jcs.107987; PMID: 22976294
- Pillai RS, Chuma S. piRNAs and their involvement in male germline development in mice. Dev Growth Differ 2012; 54:78 - 92; http://dx.doi.org/10.1111/j.1440-169X.2011.01320.x; PMID: 22221002
- Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 2009; 10:126 - 39; http://dx.doi.org/10.1038/nrm2632; PMID: 19165215
- Lau NC, Seto AG, Kim J, Kuramochi-Miyagawa S, Nakano T, Bartel DP, et al. Characterization of the piRNA complex from rat testes. Science 2006; 313:363 - 7; http://dx.doi.org/10.1126/science.1130164; PMID: 16778019
- Katakowski M, Buller B, Wang X, Rogers T, Chopp M. Functional microRNA is transferred between glioma cells. Cancer Res 2010; 70:8259 - 63; http://dx.doi.org/10.1158/0008-5472.CAN-10-0604; PMID: 20841486
- Brink PR, Valiunas V, Gordon C, Rosen MR, Cohen IS. Can gap junctions deliver?. Biochim Biophys Acta 2012; 1818:2076 - 81; http://dx.doi.org/10.1016/j.bbamem.2011.09.025; PMID: 21986484
- Wong CH, Mruk DD, Lui WY, Cheng CY. Regulation of blood-testis barrier dynamics: an in vivo study. J Cell Sci 2004; 117:783 - 98; http://dx.doi.org/10.1242/jcs.00900; PMID: 14734653
- Young JS, Guttman JA, Vaid KS, Shahinian H, Vogl AW. Cortactin (CTTN), N-WASP (WASL), and clathrin (CLTC) are present at podosome-like tubulobulbar complexes in the rat testis. Biol Reprod 2009; 80:153 - 61; http://dx.doi.org/10.1095/biolreprod.108.070615; PMID: 18799755
- Mok KW, Mruk DD, Lee WM, Cheng CY. Spermatogonial stem cells alone are not sufficient to re-initiate spermatogenesis in the rat testis following adjudin-induced infertility. Int J Androl 2012; 35:86 - 101; http://dx.doi.org/10.1111/j.1365-2605.2011.01183.x; PMID: 21696392