Abstract
This review introduces the traditionally defined anatomic compartments of the peripheral nerves based on light and electron microscopic topography and then explores the cellular and the most recent molecular basis of the different barrier functions operative in peripheral nerves. We also elucidate where, and how, the homeostasis of the normal human peripheral nerve is controlled in situ and how claudin-containing tight junctions contribute to the barriers of peripheral nerve. Also, the human timeline of the development of the barriers of the peripheral nerve is depicted. Finally, potential future therapeutic modalities interfering with the barriers of the peripheral nerve are discussed.
Introduction
Epineurium, perineurium and endoneurium are the three connective tissue compartments of the peripheral nerve (). The structure of these compartments is intimately associated with their function as shielding barriers for the impulse-conducting elements. The perineurium and its projections into the endoneurium are instrumental in forming the nerve-tissue barrier and the blood-nerve barrier.Citation1-Citation3 The autotypic junctions of Schwann cells divide the myelinating Schwann cell into compartments of compact and non-compact myelin (). The existence of the tight junction strands between perineurial cells has been demonstrated in electron microscopic studies already for four decades ago.Citation4 Characterization of the tight junction proteins in 1990s provided tools to analyze the molecular composition of perineurial tight junctions in the early years of this century.Citation22,Citation44 These studies have addressed the permeability barrier to claudin-containing junction structures in perineurium, endoneurial vasculature as well as Schwann cell autotypic junctions.
Figure 1. The three anatomical compartments of the peripheral nerve are the epineurium, the perineurium and the endoneurium. The figure demonstrates the localization and components of perineurial, endothelial and Schwann cell barriers. Cl, claudin; Occl, occluding.
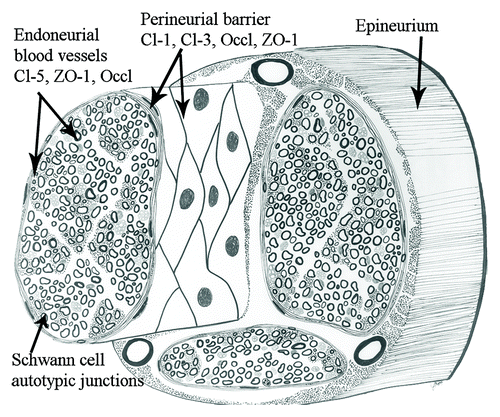
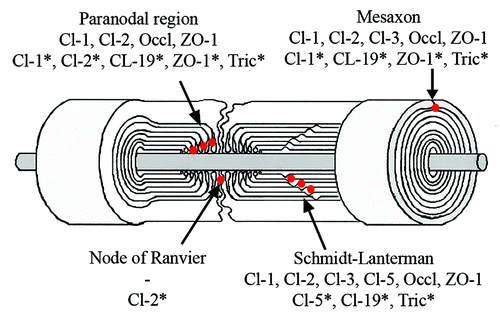
Figure 2. Composition of Schwann cell autotypic junctions. Their localization in Schwann cells is shown as red dots. *Rodents.
Sheets of cells and their tight junctions contribute to diffusion barriers in various tissues. Tight junction components include claudins, occludin, junctional adhesion protein (JAM), tricellulin and other membrane associated or scaffolding proteins such as zonula occludens (ZO)-1, -2, -3, Discs Lost-multi PDZ domain protein 1 (MUPP1) and Pals-associated tight junction protein (PATJ).Citation5 Claudin family consists of 27 members, of which four are expressed in the peripheral nerve in man and in mouse.Citation6,Citation7 In peripheral nerve, basement membrane covers perineurial cells and Schwann cells while endothelial cells are attached to basement membrane. Basement membranes limit the movement of molecules according to their size and electrical charge.
Anatomical Compartments of Peripheral Nerve
Epineurium is composed mostly of collagenous extracellular matrix which surrounds the entire nerve and its individual fascicles. It thus contributes to the tensile strength of the nerve, but does not form barriers.
Perineurium isolates groups of axon-Schwann cell units to form nerve fascicles and constitutes the main diffusion barrier between the endoneurium and the extrafascicular tissues.Citation8-Citation10 Perineurium is composed of concentric layers of large, flat perineurial cells. The number of perineurial cell layers varies according to the number and size of the fascicles in the nerve; the largest fascicles in, e.g., sciatic nerve may have 15 layers. The thickness of the perineurium also decreases toward the nerve periphery where smallest fascicles may only have one or two perineurial layers.Citation1 Pockets containing extracellular matrix are present between the neighboring perineurial cell layers. The extracellular matrix is composed of fibrillar and microfibrillar collagens and fibronectin which provide the perineurium with the ability to modulate external stretching forces thus regulating the endoneurial pressure.Citation11,Citation12 Perineurial cells are able to produce a number of extracellular matrix molecules.Citation13 Each perineurial cell layer is surrounded by prominent basement membrane which can in larger nerves be up to 500 nm thick and provide additional molecular barrier.Citation3,Citation14 All basement membranes contain type IV collagen and laminin. However, the human perineurial and Schwann cell basement membranes contain rare laminin chains: Schwann cells express laminin heavy chain α2 which was originally called merosin and in the present nomenclature is called laminin 211. Perineurial cell basement membrane contains laminin β2 chain, originally named s-laminin, which is also present in Schwann cell basement membrane but probably as a minor component. Thus, Schwann cell and perineurial basement membranes are somewhat distinct with respect to their laminin isoforms but the significance of this difference is not known.Citation15 In addition to structural proteins, basement membranes contain heparin sulfate proteoglycans which have a negative charge and thus function as extracellular molecular filters preventing diffusion of molecules larger than 12 nm in diameter.Citation16,Citation17 Perineurial cells are connected to each other by tight junction strands which are formed between the partly overlapping processes of the adjacent cells (see below).
Endoneurium contains groups of axon-Schwann cell units embedded in extracellular matrix which has thin collagen fibrils and gel-like consistency, as evaluated under preparation microscope. Axons are covered by Schwann cells which form either myelin or amyelin sheath. Myelinating Schwann cell membranes wrap around the axon resulting in multilamellar membrane structures. The myelin sheath is divided into compacted and non-compacted myelin compartments. Non-compact myelin is present in three locations: (1) immediately next to the nodes of Ranvier (paranodal region) which allow the electrical impulse to conduct quickly, (2) in Schmidt-Lanterman incisures located inside of compacted myelin sheath and (3) the inner and outer mesaxons where the volume of Schwann cell cytoplasm is increased compared with the areas of compacted myelin. Several types of autotypic junctions, including tight junctions, participate in the adhesion of apposed membrane lamellae in the sites of non-compacted myelin ().Citation1
The Functional Barriers of Peripheral Nerve
Perineurial barrier
Perineurium forms a metabolically active diffusion barrier in the peripheral nerve.Citation8-Citation10 Perineurium functions to maintain the homeostasis of the endoneurium, including the constant intrafascicular pressure. Perineurial barrier has three structural and functional components: (1) Basement membranes surrounding each perineurial cell layer (see above), (2) tight junctions between the neighboring perineurial cells and (3) active transcytotic transport through the perineurial cells.
Strands of tight junctions have been visualized in ultrastructural studies at the interdigitating perineurial cell borders. Tracer and electrophysiological studies have shown that perineurium constitutes a tight but selective barrier.Citation2,Citation4,Citation18-Citation21 The molecular composition of human perineurial tight junctions has been studied by Pummi and coworkers who detected occludin, ZO-1 and claudin-1 and -3 adult human perineurium.Citation22 Thus, the perineurial tight junctions can contain at least three different claudin dimers: claudin-1/-1, claudin-1/-3 and claudin-3/-3. Since this report, several new members of the claudin family and other tight junction proteins have been characterized but they have not been mapped to human perineurium so far.
Transcytosis, as evidenced by the presence of numerous pinocytotic vesicles, provides an alternative route through perineurium. Ultrastructural studies have shown numerous pinocytotic vesicles in perineurial cells, but this phenomenon has not been studied at the molecular level.Citation14,Citation23 Thus, it is not known whether the traffic is directed into or out of the nerve or which receptors or caveolins are involved. Molecules can also be actively transported across the perineurium via specific membrane receptors. Of various receptors studied only Glucose transporter 1 (GLUT -1) has been localized to human and rat perineurium.Citation9
Developmental aspects of perineurium
During the fetal life the diameter of peripheral nerves increases due to the growth of fascicles in size and in number, as well as due to the synthesis of extracellular matrix. The number and the thickness of perineurial cell layers increase and intrafascicular septae become more visible.
In human, development of the perineurial diffusion barrier takes place relatively late. During the first trimester, perineurium is composed of one or two layers of perineurial cells and thin intrafascicular septae can be distinguished. Morphological studies have shown the first evidence of perineurial tight junctions at fetal weeks 12−14.Citation15,Citation24,Citation25 Claudin-1 is the first tight junction protein which has been detected in perineurium around 11 weeks as punctate labeling.Citation22 Claudin-3, ZO-1 and occludin are present at the fetal week 22, although the labeling pattern for ZO-1 and occludin is still punctate. The tight junction strands with linear distribution of occludin, ZO-1 and claudin-1 and -3 are detectable at 35 fetal weeks, but the labeling of tight junctions is still not as continuous as in adults.Citation22 The maturation of the perineurial tight junctions takes place along with the maturation of the extracellular diffusion barrier. The basement membrane components type IV collagen and laminins appear concomitantly with the expression of tight junction proteins and basement membranes become continuous at the end of the third trimester.Citation15 The maturation of the perineurial diffusion barrier also takes place along with the metabolical activity of the perineurial cells since the expression of glucose transporter 1 (GLUT-1) can be first detected in the perineurium between fetal weeks 15 and 17.Citation10
Blood-nerve barrier
Blood-nerve barrier is located in the blood vessel walls of the endoneurial vasculature. Here tight junctions are found between endothelial cells and between pericytes. Endoneurial blood vessels isolate the endoneurim from the circulating blood thus preventing uncontrollable molecule and ion leak from circulatory system to peripheral nerve. Blood-nerve barrier is thus analogous to the blood-brain barrier. The blood vessels entering the endoneurium through perineurium are first covered by a sleeve of perineurial cells.Citation10 The endothelium of endoneurial blood vessels is attached to the continuous basement membrane. The pericytes are also covered by continuous basement membrane. Endoneurial capillaries differ ultrastructurally from epi- and perineurial vessels since the endoneurial capillaries are lined by non-fenestrated endothelial cells containing few plasmalemmal vesicles. Also this fact suggests that endoneurial vessels have tighter permeability characteristics than epineurial vessels.Citation17
The barrier properties of the endoneurial blood vessels have been studied mainly in animal models. The endothelial cell cultures from rat sciatic nerves have been shown to develop higher transendothelial electrical resistance (TEER) than HUVEC cultures, which is an indication of well-developed tight junctions.Citation26 The endothelial cells express occludin, ZO-1, ZO-2, claudin-5, claudin-12 and JAM in culture, and the same claudins and occludin have been shown in rat sciatic nerve vivo.Citation26 Claudin-5 can be considered as a sign of a tight permeability barrier. Downregulation of claudin-5 and ZO-1 in endothelium has been shown in chronic inflammatory demyelinating neuropathy.Citation27
The endoneurial pericytes have been shown to be essential for the function of the blood-nerve-barrier.Citation28 In vitro, cultured pericytes are able to express mRNA for occludin, ZO-1, ZO-2, JAM and claudin-12 but not claudin-5.Citation29 It has also been proposed that occludin localized at the pericyte–pericyte boundaries might be the key molecule for the mechanical stability of microvessels and the low paracellular permeability.Citation29 The growth factors secreted by pericytes regulate the claudin-5 expression in endothelial cells resulting stronger or weaker barrier.Citation30,Citation31
In analogy to perineurial cells, pericytes actively transfer molecules through cells. Pericytes express several barrier-associated transport molecules, such as glucose transporter GLUT-1, ATP-binding cassette (ABC) transporters ABCG2 and MRP1 and phosphoglycolate phosphatase p-gp.Citation29 It is of interest to note that epineurial blood vessels which do not contribute to the blood-nerve barrier do not express GLUT-1. 9Pericytes have been attributed to stem cell characteristics, and vascular wall stem cells have even been suggested to be the origin of tumor Schwann cells in cutaneous neurofibromas.Citation32 Cutaneous neurofibromas are benign tumors containing all cell types and elements of peripheral nerves.Citation33 Neurofibromas have been widely studied also for understanding the biology of peripheral nerve connective tissue cells.Citation34-Citation36
Schwann cell autotypic junctions
In analogy to cells of simple epithelia whose polarity is defined by localization of junctional proteins, a myelinating cell is divided to compacted and non-compacted areas by autotypic junctions between the membranes of the same Schwann cell (). The exact function of Schwann cell autotypic junctions is not known but it has been suggested that they separate the outer membrane and the extracellular space from the compact myelin and also provide the membrane associations for mechanical strength.Citation37 Claudin-containing junctions may also have an effect on nerve conduction, as suggested by the results from claudin-19 knockout mouse.Citation38 These autotypic junctions include adherens, tight and gap junctions but there is evidence that the structure and molecular composition of these junctions differs from those of epithelial cell junctions.Citation37,Citation39-Citation41 Cytoplasm-rich areas of non-compact myelin, mainly Schmidt-Lanterman incisures, form helical channel during myelination and provide diffusion pathway bringing outer membrane lamellae closer to each other despite barrier function.Citation42 Gap junctions in Schmidt-Lanterman incisures and paranodal regions provide quick radial pathway for small molecules and ions through membrane lamellae.Citation42 Other areas of tight junctions both in myelinated and non-myelinated Schwann cells have been mapped to node of Ranvier and inner and outer mesaxons.
The composition of the Schwann cell autotypic junctions has mainly been studied in rodents which express claudin-1,-2,-5,-19, ZO-1 and ZO-2 in Schwann cells ().Citation37,Citation38,Citation41-Citation43 Different claudins were associated with MUPP1 and PATJ. Claudin-19 is highly expressed in mouse peripheral nerve and localized to Schmidt-Lanterman incisures, paranodal region and inner and outer mesaxons.Citation38 Tricellulin has recently been shown in paranodal region, Schmidt-Lanterman incisures and mesaxons, but the expression of tricellulin or claudin-19 have not been studied in human peripheral nerve.Citation43
Table1. Tight junction proteins in peripheral nerve
Expression of mRNA for claudin-1,-2,-3, -5,-9 and -11 has been detected in adult human peripheral nerve.Citation44 Tight junction components claudin-1, claudin-2 and ZO-1, as well as E-cadherin, a component of adherens junctions, were localized in paranodal region. Schmidt-Lanterman incisures were positive for claudin-1,-2, -3 and -5, occludin, ZO-1 and E-cadherin. Claudin-1,-2,and -3, ZO-1, occludin and E-cadherin, but not claudin-5, were localized to mesaxons ().Citation44
Tight junction components have also been examined in developing endoneurium of human sciatic nerves. During the first trimester, tight junction components were not reliably detectable. On fetal week 22, antibodies for claudin-1, claudin-3, occludin and ZO-1 showed punctate and linear labeling suggesting mesaxonal localization. At the end of third trimester (week 37) labeling pattern of all four tight junction components was linear and occasionally claudin-1 and claudin-3 labeling could be mapped to Schmidt-Lanterman incisures as a sign of myelination.Citation44
Several claudin mouse models have been reported but only two of them focus on claudin-19 in peripheral nerve. Claudin-19 knockout resulted in abnormal gait because of peripheral nervous system defect and specifically Schwann cell barrier defect, but did not show defects in kidney.Citation38 In another mouse model the claudin-19 gene was silenced by siRNA technique and no dysfunction or morphological abnormalities in peripheral were detected.Citation45 Thus the functional significance of claudin-19 in peripheral nerve cannot be concluded.
Tight junctions of epithelial cells have been reported to participate in membrane trafficking and vesicle transport, and tight junction proteins are in close contact with other proteins guiding traffic to apical or basolateral membranes.Citation46 It can be speculated that tight junction proteins in autotypic junctions may have similar functions in membrane and vesicle trafficking.
To conclude, myelinating cells have distinct structures, including autotypic junctions, dense membrane lamellae, paranodal regions and Schmidt-Lanterman incisures. Various events which disturb function or integrity of myelinating cell structure can lead to neuronal defects.Citation38,Citation47
Clinical Aspects of the Peripheral Nerve Barriers
While the perineurial barrier serves as protection, it simultaneously inhibits delivery of analgesic drugs to peripheral nerve. Rodent studies have shown that hypertonic saline transiently opens the barrier allowing also hydrophilic drugs to reach their target, such as opioid receptors.Citation48,Citation49 This leakage has been shown to be regulated by metalloproteinase activation and claudin-1 downregulation.Citation50 These studies also establish the function of claudin-1 in the perineurium as the major sealing component, which could be modulated to facilitate drug delivery or, potentially, reseal the barrier under pathological conditions. When studied further, the ability to regulate perineurial diffusion by downregulating claudin-1 may potentially be an important clinical application of claudin research.
Perineurium has an important role in the nerve repair process after trauma. Tight and gap junctions have been elucidated in several experimental studies during recovery of perineurium. The traditional and most widely used peripheral nerve injury model is crush injury which leads to opening of the perineurial barrier and distortion of the neural homeostasis. A more sophisticated injury model is the perineurial window model, in which excision of perineum induces focal demyelination of the remaining nerve fibers in the center of nerve fascicles. Detectable signs of recovery process, such as thin perineurial cell layer and first evidence of tight junctions appear within one week, and by three weeks perineurium has tight junction strands and gap junctions. Perineurial window model emphasizes the role of tight junctions in perineurial repair process.Citation51-Citation53 Corresponding results have been shown in the nerve injury model in which ligating rat sciatic nerve for 24h resulted in the recovery of claudin-1 expression in perineurium within two days. Gap junctions appeared rather late, within five days and the nerve recovery was accomplished during seven days. Endoneurial blood vessels recovered quickly and full function of blood-nerve barrier was achieved during five days.Citation54
Expression of claudin-1 belongs to constant characteristics of perineurial cells, and claudin-1 can be used as a marker for neoplastic perineurial cells, along with Glut-1 and EMA. Claudin-1 is expressed by benign and malignant perineurial tumor cells.Citation3
Studies on tight junction proteins of nervous system are mainly focused on the blood-brain barrier in conditions such as Alzheimer disease, brain stroke, epilepsy and Parkinson disease. Failures in structure or function of blood-brain barrier may result in the development of neurological disease.Citation55 However, only a few diseases have been reported concerning dysfunction of tight junction in peripheral nerve. The importance of pericytes is emphasized in diabetic neuropathy in which pericyte degeneration and loss with thickening of basement membranes are the main features.Citation28 Pericytes in neural blood vessels regulate the claudin-5 expression in endothelial cells. Decreased claudin-5 expression in endothelial cells weakens blood-nerve barrier and may lead to demyelinating polyradiculoneuropathy.Citation27,Citation30
Human mutations in claudin-1, -14, -16 and -19 genes have been discovered but none of these have led to symptomps of peripheral nerve.Citation56-Citation61 It is of interest to note, that mutation in human claudin-19 gene did not result in any defects in peripheral nervous system even though claudin-19 is strongly expressed in mouse Schwann autotypic junctions.Citation38,Citation59
Conclusions
Studies on neural barriers have mainly concentrated on blood-brain and blood-nerve barriers while knowledge of functions and molecular basis of perineurial barrier are still sparse. Thus, there are many unanswered questions concerning, e.g., the perineurial transcytotic transport, perineurial receptors and even the exact composition of tight junctions. Only a few claudin types have been mapped to perineurium and there may still be tight junction components which have not been studied for this respect. This may be due to the fact that the unique structure and function of the perineurium has not been widely recognized. Instead, perineurium seems to be overlooked in most periphral nerve studies in which the main focus is in the impulse-conducting elements.
Studying human peripheral nerve is always more or less challenging because the samples are not readily available. Animal models have often been good alternatives but using animal models one has to keep in mind that e.g., expression of different claudin types varies between species and conclusions from animal studies to human biology and diseases should be drawn cautiously.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
References
- Mizisin AP, Weerasuriya A. Homeostatic regulation of the endoneurial microenvironment during development, aging and in response to trauma, disease and toxic insult. Acta Neuropathol 2011; 121:291 - 312; http://dx.doi.org/10.1007/s00401-010-0783-x; PMID: 21136068
- Thomas PK. The connective tissue of peripheral nerve: an electron microscope study. J Anat 1963; 97:35 - 44; PMID: 13981107
- Piña-Oviedo S, Ortiz-Hidalgo C. The normal and neoplastic perineurium: a review. Adv Anat Pathol 2008; 15:147 - 64; http://dx.doi.org/10.1097/PAP.0b013e31816f8519; PMID: 18434767
- Reale E, Luciano L, Spitznas M. Freeze-fracture faces of the perineurial sheath of the rabbit sciatic nerve. J Neurocytol 1975; 4:261 - 70; http://dx.doi.org/10.1007/BF01102112; PMID: 1133587
- Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol 2009; 1:a002584; http://dx.doi.org/10.1101/cshperspect.a002584; PMID: 20066090
- Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol 1998; 141:1539 - 50; http://dx.doi.org/10.1083/jcb.141.7.1539; PMID: 9647647
- Mineta K, Yamamoto Y, Yamazaki Y, Tanaka H, Tada Y, Saito K, et al. Predicted expansion of the claudin multigene family. FEBS Lett 2011; 585:606 - 12; http://dx.doi.org/10.1016/j.febslet.2011.01.028; PMID: 21276448
- Shanthaveerappa TR, Bourne GH. The ‘perineural epithelium’, a metabolically active, continuous, protoplasmic cell barrier surrounding peripheral nerve fasciculi. J Anat 1962; 96:527 - 37; PMID: 13976799
- Allt G, Lawrenson JG. The blood-nerve barrier: enzymes, transporters and receptors--a comparison with the blood-brain barrier. Brain Res Bull 2000; 52:1 - 12; http://dx.doi.org/10.1016/S0361-9230(00)00230-6; PMID: 10779695
- Muona P, Jaakkola S, Salonen V, Peltonen J. Expression of glucose transporter 1 in adult and developing human peripheral nerve. Diabetologia 1993; 36:133 - 40; http://dx.doi.org/10.1007/BF00400694; PMID: 7681417
- Peltonen J, Jaakkola S, Hsiao LL, Timpl R, Chu ML, Uitto J. Type VI collagen. In situ hybridizations and immunohistochemistry reveal abundant mRNA and protein levels in human neurofibroma, schwannoma and normal peripheral nerve tissues. Lab Invest 1990; 62:487 - 92; PMID: 2332972
- Muona P, Jaakkola S, Zhang RZ, Pan TC, Pelliniemi L, Risteli L, et al. Hyperglycemic glucose concentrations up-regulate the expression of type VI collagen in vitro. Relevance to alterations of peripheral nerves in diabetes mellitus. Am J Pathol 1993; 142:1586 - 97; PMID: 8494053
- Jaakkola S, Peltonen J, Uitto JJ. Perineurial cells coexpress genes encoding interstitial collagens and basement membrane zone components. J Cell Biol 1989; 108:1157 - 63; http://dx.doi.org/10.1083/jcb.108.3.1157; PMID: 2921281
- Gamble HJ, Eames RA. An electron microscope study of the connective tissues of human peripheral nerve. J Anat 1964; 98:655 - 63; PMID: 14229996
- Jaakkola S, Savunen O, Halme T, Uitto J, Peltonen J. Basement membranes during development of human nerve: Schwann cells and perineurial cells display marked changes in their expression profiles for laminin subunits and beta 1 and beta 4 integrins. J Neurocytol 1993; 22:215 - 30; http://dx.doi.org/10.1007/BF01246360; PMID: 8478643
- Linker A, Hovingh P, Kanwar YS, Farquhar MG. Characterization of heparan sulfate isolated from drug glomerular basement membranes. Lab Invest 1981; 44:560 - 5; PMID: 6453253
- Bush MS, Allt G. Blood-nerve barrier: distribution of anionic sites on the endothelial plasma membrane and basal lamina. Brain Res 1990; 535:181 - 8; http://dx.doi.org/10.1016/0006-8993(90)91599-C; PMID: 1705854
- Kristensson K, Olsson Y. The perineurium as a diffusion barrier to protein tracers. Differences between mature and immature animals. Acta Neuropathol 1971; 17:127 - 38; http://dx.doi.org/10.1007/BF00687488; PMID: 5101596
- Beamish NG, Stolinski C, Thomas PK, King RH, Oldfors A. A freeze-fracture study of the perineurium in normal and protein-deprived rats. APMIS 1991; 99:941 - 55; http://dx.doi.org/10.1111/j.1699-0463.1991.tb01282.x; PMID: 1930967
- Ghabriel MN, Jennings KH, Allt G. Diffusion barrier properties of the perineurium: an in vivo ionic lanthanum tracer study. Anat Embryol (Berl) 1989; 180:237 - 42; http://dx.doi.org/10.1007/BF00315882; PMID: 2596704
- Todd BA, Inman C, Sedgwick EM, Abbott NJ. Ionic permeability of the opossum sciatic nerve perineurium, examined using electrophysiological and electron microscopic techniques. Brain Res 2000; 867:223 - 31; http://dx.doi.org/10.1016/S0006-8993(00)02312-X; PMID: 10837817
- Pummi KP, Heape AM, Grénman RA, Peltonen JT, Peltonen SA. Tight junction proteins ZO-1, occludin, and claudins in developing and adult human perineurium. J Histochem Cytochem 2004; 52:1037 - 46; http://dx.doi.org/10.1369/jhc.3A6217.2004; PMID: 15258179
- Oldfors A. Permeability of the perineurium of small nerve fascicles: an ultrastructural study using ferritin in rats. Neuropathol Appl Neurobiol 1981; 7:183 - 94; http://dx.doi.org/10.1111/j.1365-2990.1981.tb00088.x; PMID: 7242847
- Gamble HJ. Further electron microscope studies of human foetal peripheral nerves. J Anat 1966; 100:487 - 502; PMID: 6007459
- Gamble HJ, Breathnach AS. An electron-microscope study of human foetal peripheral nerves. J Anat 1965; 99:573 - 84; PMID: 5857090
- Sano Y, Shimizu F, Nakayama H, Abe M, Maeda T, Ohtsuki S, et al. Endothelial cells constituting blood-nerve barrier have highly specialized characteristics as barrier-forming cells. Cell Struct Funct 2007; 32:139 - 47; http://dx.doi.org/10.1247/csf.07015; PMID: 18057801
- Kanda T, Numata Y, Mizusawa H. Chronic inflammatory demyelinating polyneuropathy: decreased claudin-5 and relocated ZO-1. J Neurol Neurosurg Psychiatry 2004; 75:765 - 9; http://dx.doi.org/10.1136/jnnp.2003.025692; PMID: 15090575
- Giannini C, Dyck PJ. Basement membrane reduplication and pericyte degeneration precede development of diabetic polyneuropathy and are associated with its severity. Ann Neurol 1995; 37:498 - 504; http://dx.doi.org/10.1002/ana.410370412; PMID: 7717686
- Shimizu F, Sano Y, Maeda T, Abe MA, Nakayama H, Takahashi R, et al. Peripheral nerve pericytes originating from the blood-nerve barrier expresses tight junctional molecules and transporters as barrier-forming cells. J Cell Physiol 2008; 217:388 - 99; http://dx.doi.org/10.1002/jcp.21508; PMID: 18543246
- Shimizu F, Sano Y, Abe MA, Maeda T, Ohtsuki S, Terasaki T, et al. Peripheral nerve pericytes modify the blood-nerve barrier function and tight junctional molecules through the secretion of various soluble factors. J Cell Physiol 2011; 226:255 - 66; http://dx.doi.org/10.1002/jcp.22337; PMID: 20665675
- Shimizu F, Sano Y, Saito K, Abe MA, Maeda T, Haruki H, et al. Pericyte-derived glial cell line-derived neurotrophic factor increase the expression of claudin-5 in the blood-brain barrier and the blood-nerve barrier. Neurochem Res 2012; 37:401 - 9; http://dx.doi.org/10.1007/s11064-011-0626-8; PMID: 22002662
- Friedrich RE, Holstein AF, Middendorff R, Davidoff MS. Vascular wall cells contribute to tumourigenesis in cutaneous neurofibromas of patients with neurofibromatosis type 1. A comparative histological, ultrastructural and immunohistochemical study. Anticancer Res 2012; 32:2139 - 58; PMID: 22593502
- Jouhilahti EM, Peltonen S, Callens T, Jokinen E, Heape AM, Messiaen L, et al. The development of cutaneous neurofibromas. Am J Pathol 2011; 178:500 - 5; http://dx.doi.org/10.1016/j.ajpath.2010.10.041; PMID: 21281783
- Jouhilahti EM, Peltonen S, Heape AM, Peltonen J. The pathoetiology of neurofibromatosis 1. Am J Pathol 2011; 178:1932 - 9; http://dx.doi.org/10.1016/j.ajpath.2010.12.056; PMID: 21457932
- Peltonen J, Jaakkola S, Lebwohl M, Renvall S, Risteli L, Virtanen I, et al. Cellular differentiation and expression of matrix genes in type 1 neurofibromatosis. Lab Invest 1988; 59:760 - 71; PMID: 2462129
- Jaakkola S, Peltonen J, Riccardi V, Chu ML, Uitto J. Type 1 neurofibromatosis: selective expression of extracellular matrix genes by Schwann cells, perineurial cells, and fibroblasts in mixed cultures. J Clin Invest 1989; 84:253 - 61; http://dx.doi.org/10.1172/JCI114148; PMID: 2500456
- Poliak S, Matlis S, Ullmer C, Scherer SS, Peles E. Distinct claudins and associated PDZ proteins form different autotypic tight junctions in myelinating Schwann cells. J Cell Biol 2002; 159:361 - 72; http://dx.doi.org/10.1083/jcb.200207050; PMID: 12403818
- Miyamoto T, Morita K, Takemoto D, Takeuchi K, Kitano Y, Miyakawa T, et al. Tight junctions in Schwann cells of peripheral myelinated axons: a lesson from claudin-19-deficient mice. J Cell Biol 2005; 169:527 - 38; http://dx.doi.org/10.1083/jcb.200501154; PMID: 15883201
- Arroyo EJ, Scherer SS. On the molecular architecture of myelinated fibers. Histochem Cell Biol 2000; 113:1 - 18; http://dx.doi.org/10.1007/s004180050001; PMID: 10664064
- Mugnaini E, Schnapp B. Possible role of zonula occludens of the myelin sheath in demyelinating conditions. Nature 1974; 251:725 - 7; http://dx.doi.org/10.1038/251725a0; PMID: 4610402
- Fannon AM, Sherman DL, Ilyina-Gragerova G, Brophy PJ, Friedrich VL Jr., Colman DR. Novel E-cadherin-mediated adhesion in peripheral nerve: Schwann cell architecture is stabilized by autotypic adherens junctions. J Cell Biol 1995; 129:189 - 202; http://dx.doi.org/10.1083/jcb.129.1.189; PMID: 7698985
- Balice-Gordon RJ, Bone LJ, Scherer SS. Functional gap junctions in the schwann cell myelin sheath. J Cell Biol 1998; 142:1095 - 104; http://dx.doi.org/10.1083/jcb.142.4.1095; PMID: 9722620
- Kikuchi S, Ninomiya T, Tatsumi H, Sawada N, Kojima T. Tricellulin is expressed in autotypic tight junctions of peripheral myelinating Schwann cells. J Histochem Cytochem 2010; 58:1067 - 73; http://dx.doi.org/10.1369/jhc.2010.956326; PMID: 21097846
- Alanne MH, Pummi K, Heape AM, Grènman R, Peltonen J, Peltonen S. Tight junction proteins in human Schwann cell autotypic junctions. J Histochem Cytochem 2009; 57:523 - 9; http://dx.doi.org/10.1369/jhc.2009.951681; PMID: 19153196
- Hou J, Renigunta A, Gomes AS, Hou M, Paul DL, Waldegger S, et al. Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc Natl Acad Sci U S A 2009; 106:15350 - 5; http://dx.doi.org/10.1073/pnas.0907724106; PMID: 19706394
- Zahraoui A, Louvard D, Galli T. Tight junction, a platform for trafficking and signaling protein complexes. J Cell Biol 2000; 151:F31 - 6; http://dx.doi.org/10.1083/jcb.151.5.F31; PMID: 11086016
- Gow A, Southwood CM, Li JS, Pariali M, Riordan GP, Brodie SE, et al. CNS myelin and sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell 1999; 99:649 - 59; http://dx.doi.org/10.1016/S0092-8674(00)81553-6; PMID: 10612400
- Rittner HL, Amasheh S, Moshourab R, Hackel D, Yamdeu RS, Mousa SA, et al. Modulation of tight junction proteins in the perineurium to facilitate peripheral opioid analgesia. Anesthesiology 2012; 116:1323 - 34; http://dx.doi.org/10.1097/ALN.0b013e318256eeeb; PMID: 22534246
- Hackel D, Brack A, Fromm M, Rittner HL. Modulation of tight junction proteins in the perineurium for regional pain control. Ann N Y Acad Sci 2012; 1257:199 - 206; http://dx.doi.org/10.1111/j.1749-6632.2012.06499.x; PMID: 22671607
- Hackel D, Krug SM, Sauer RS, Mousa SA, Böcker A, Pflücke D, et al. Transient opening of the perineurial barrier for analgesic drug delivery. Proc Natl Acad Sci U S A 2012; 109:E2018 - 27; http://dx.doi.org/10.1073/pnas.1120800109; PMID: 22733753
- Ohta M, Okajima S, Hirakawa H, Tokunaga D, Fujiwara H, Oda R, et al. Expression of tight and gap junctional proteins in the perineurial window model of the rat sciatic nerve. Int J Neurosci 2005; 115:1469 - 81; http://dx.doi.org/10.1080/00207450591001871; PMID: 16162451
- Nesbitt JA, Acland RD. Histopathological changes following removal of the perineurium. J Neurosurg 1980; 53:233 - 8; http://dx.doi.org/10.3171/jns.1980.53.2.0233; PMID: 7431062
- Sugimoto Y, Takayama S, Horiuchi Y, Toyama Y. An experimental study on the perineurial window. J Peripher Nerv Syst 2002; 7:104 - 11; http://dx.doi.org/10.1046/j.1529-8027.2002.02017.x; PMID: 12090296
- Hirakawa H, Okajima S, Nagaoka T, Takamatsu T, Oyamada M. Loss and recovery of the blood-nerve barrier in the rat sciatic nerve after crush injury are associated with expression of intercellular junctional proteins. Exp Cell Res 2003; 284:196 - 210; http://dx.doi.org/10.1016/S0014-4827(02)00035-6; PMID: 12651153
- Bednarczyk J, Lukasiuk K. Tight junctions in neurological diseases. Acta Neurobiol Exp (Wars) 2011; 71:393 - 408; PMID: 22237490
- Hadj-Rabia S, Baala L, Vabres P, Hamel-Teillac D, Jacquemin E, Fabre M, et al. Claudin-1 gene mutations in neonatal sclerosing cholangitis associated with ichthyosis: a tight junction disease. Gastroenterology 2004; 127:1386 - 90; http://dx.doi.org/10.1053/j.gastro.2004.07.022; PMID: 15521008
- Thorleifsson G, Holm H, Edvardsson V, Walters GB, Styrkarsdottir U, Gudbjartsson DF, et al. Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nat Genet 2009; 41:926 - 30; http://dx.doi.org/10.1038/ng.404; PMID: 19561606
- Wilcox ER, Burton QL, Naz S, Riazuddin S, Smith TN, Ploplis B, et al. Mutations in the gene encoding tight junction claudin-14 cause autosomal recessive deafness DFNB29. Cell 2001; 104:165 - 72; http://dx.doi.org/10.1016/S0092-8674(01)00200-8; PMID: 11163249
- Konrad M, Schaller A, Seelow D, Pandey AV, Waldegger S, Lesslauer A, et al. Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am J Hum Genet 2006; 79:949 - 57; http://dx.doi.org/10.1086/508617; PMID: 17033971
- Weber S, Schneider L, Peters M, Misselwitz J, Rönnefarth G, Böswald M, et al. Novel paracellin-1 mutations in 25 families with familial hypomagnesemia with hypercalciuria and nephrocalcinosis. J Am Soc Nephrol 2001; 12:1872 - 81; PMID: 11518780
- Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, et al. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 1999; 285:103 - 6; http://dx.doi.org/10.1126/science.285.5424.103; PMID: 10390358
- Morita K, Sasaki H, Furuse M, Tsukita S. Claudin-5/Tmvcf Constitutes Tight Junction Strands in Endothelial Cells. Rockefeller University Press: J Cell Biol, 1999:185-94.