Figures & data
Figure 1 The combined Anti retroviral therapy regimens. ∗ABC: Abacavir; AZT: Zidovudine; d4T: Stavudine; ddI: Didanosine; EFV: Efavirenz; FTC: Emtricitabine; LPV: Lopinavir; NPV: Nevirapine, r: ritonavir; TDF: Tenofovir; 3TC: Lamivudine.
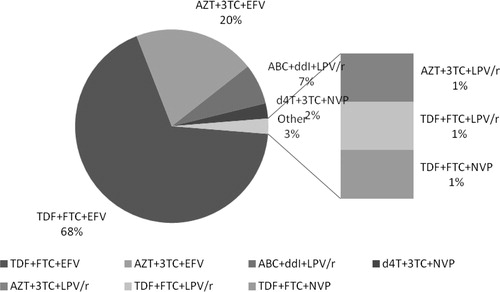
Table 1 The baseline demographic, clinical and laboratory characteristics of 118 adult HIV Positive patients attending CTC at Bugando with ARV and anti-TB Co-treatment.
Figure 2 Distribution of ARV plasma levels as sub-therapeutic, Therapeutic or supra-therapeutic among 118 adult HIV patients cotreated with TB drugs. ∗ARV: Antiretroviral; EFV: Efavirenz; HIV: Human immunodeficiency virus; LPV: Lopinavir; NVP: Nevirapine; TB: Tuberculosis.
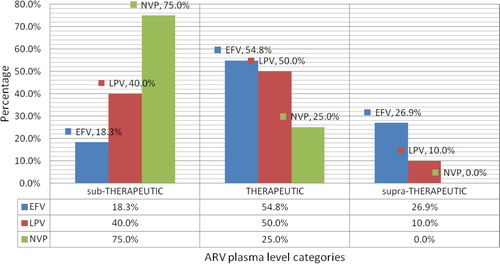
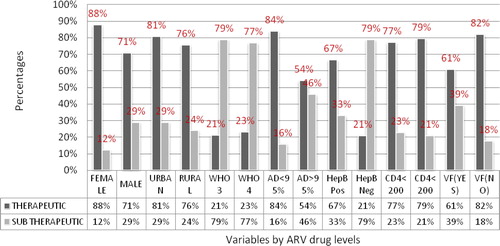
Figure 3 Distribution of variables by plasma ARV drug levels as sub-therapeutic or therapeutic. ∗AD < 95%: Adherence level of less than 95%; AD > 95%: Adherence level of more than 95%; ARV: Antiretroviral; HepB pos: Hepatitis B positive; HepB neg: Hepatitis B positive; VF: virological failure; WHO: world health organization; WHO 3: WHO clinical stage 3; WHO 4: WHO clinical stage 4.