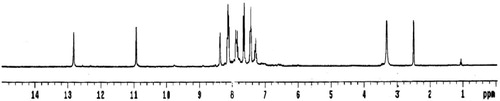
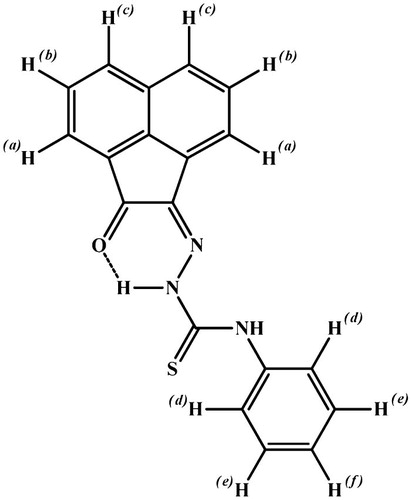
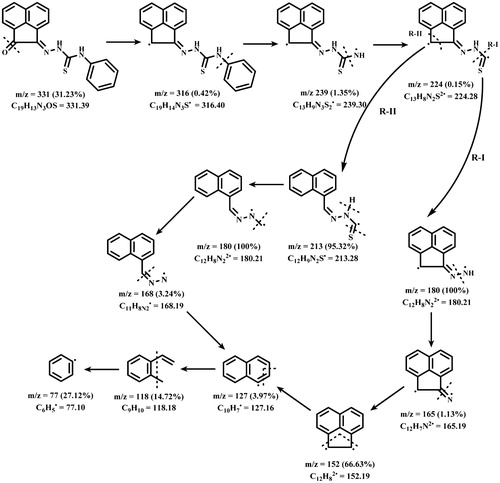
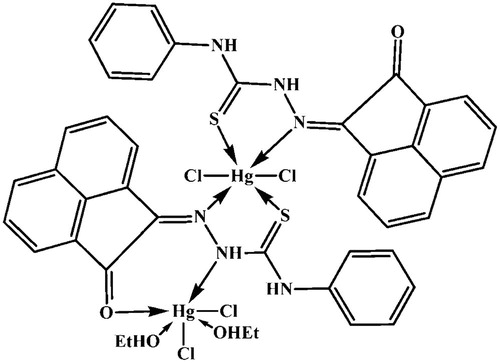
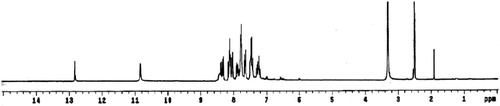
Figures & data
Table 1 Analytical and physical data of APTH and its complexes.
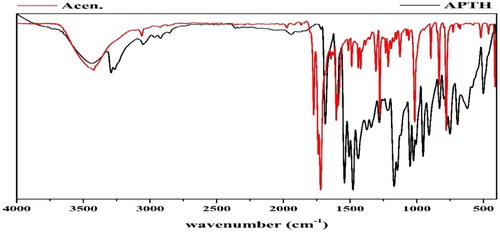
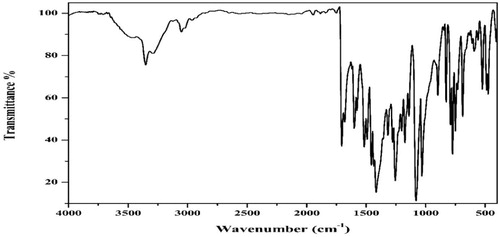
Table 2 Infrared spectral date of APTH and its metal complexes in KBr.
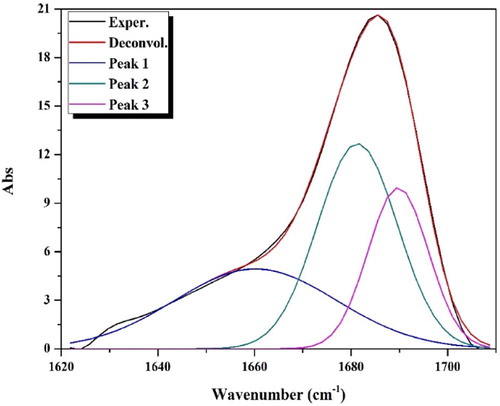
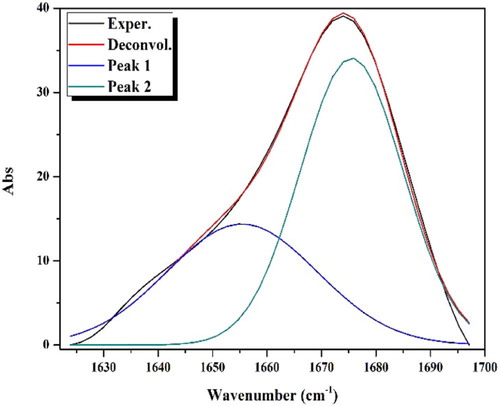
Table 3 Deconvolution analysis parameters of the APTH IR spectrum in the range 1710–1620 cm−1 (R2 = 0.9986).
Table 4 Deconvolution analysis parameters of the [Cd2(APTH)Cl4] IR spectrum in the range 1700–1620 cm−1 (R2 = 0.9966).