Figures & data
Fig. 1 SEM images of MASTL before (A) and after (B) the adsorption of dye; FT-IR spectra of MASTL before (C) and after (D) the adsorption of dye; typical N2 adsorption-desorption isotherm of MASTL (E) and UV spectra before and after the adsorption of dye onto MASTL (F).
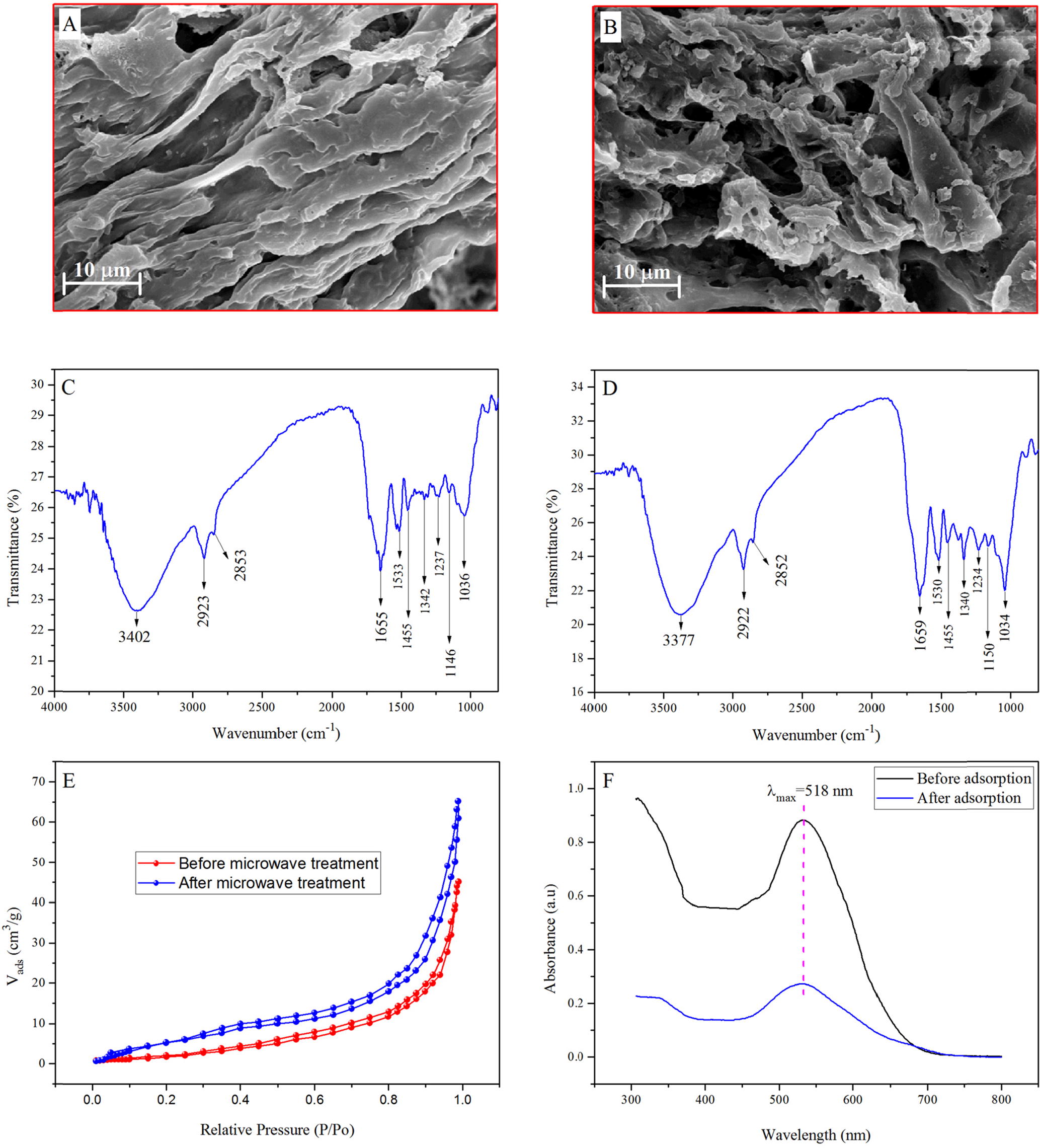
Fig. 2 Point of zero charge of MASTL (A) and the effect of the solution pH (B), at m/V = 1.0 g/L, C[EBT]initial = 60 mg/L, t = 24 h, and T = 25 °C; the effect of solid content (C), at pH = 2.0, C[EBT]initial = 60 mg/L, t = 24 h, and T = 25 °C; the effect of contact time (D), at pH = 2.0, m/V = 1.0 g/L, C[EBT]initial = 60 mg/L, and T = 25 °C.
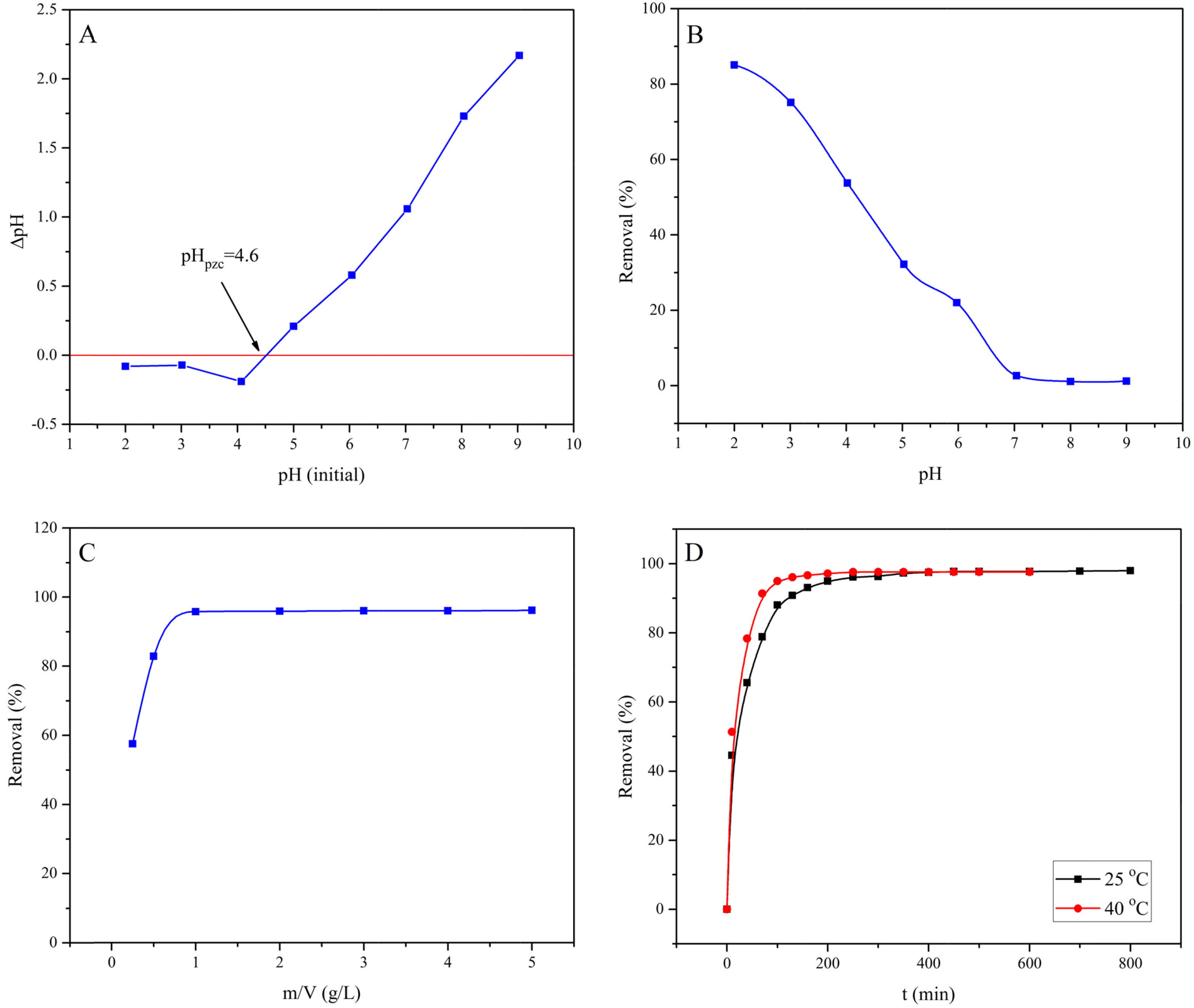
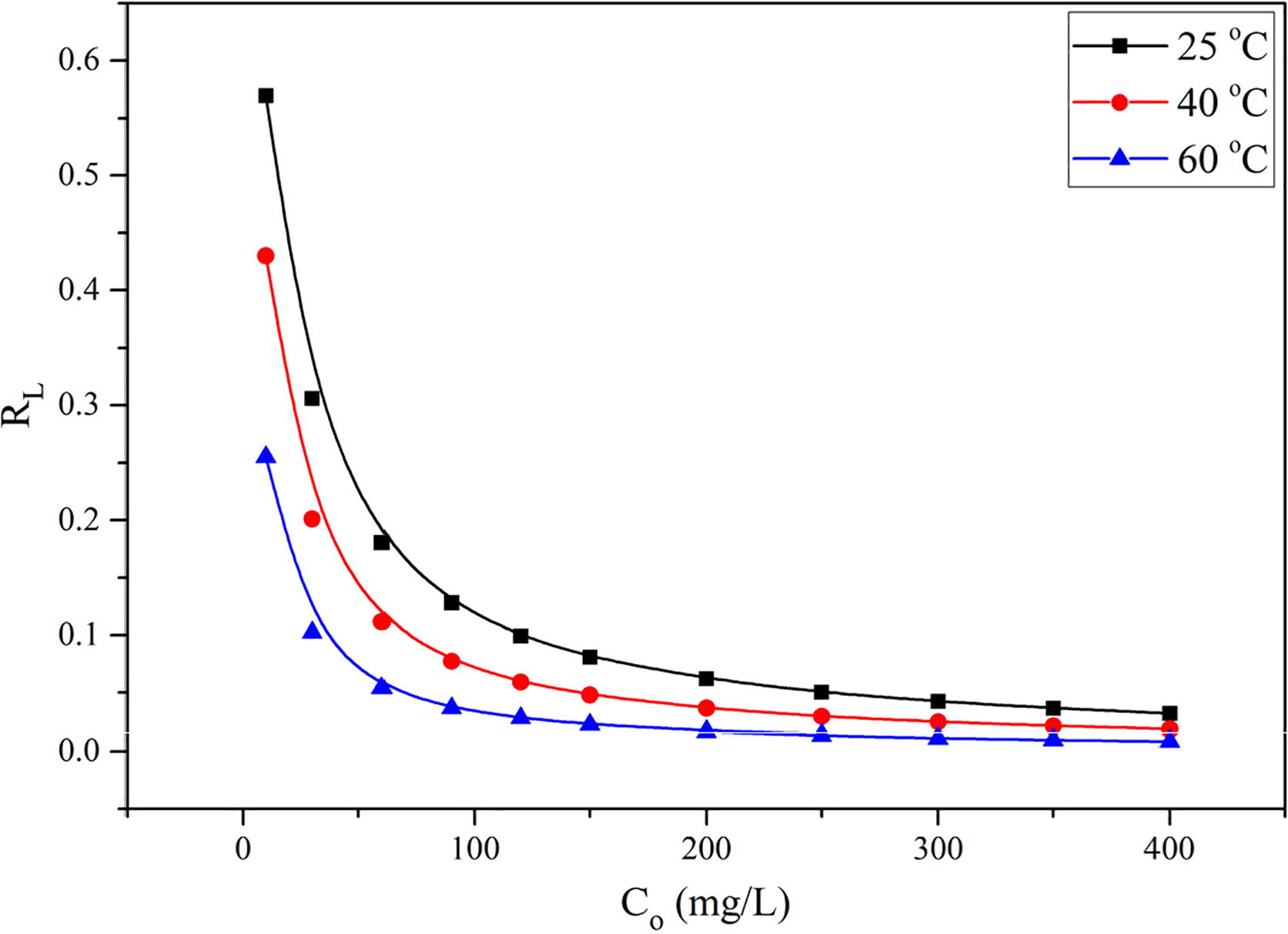
Fig. 3 Effect of temperature and initial concentration of EBT on the percent adsorption (A) and adsorption per unit mass (B), at pH = 2.0, m/V = 1.0 g/L, and t = 24 h.
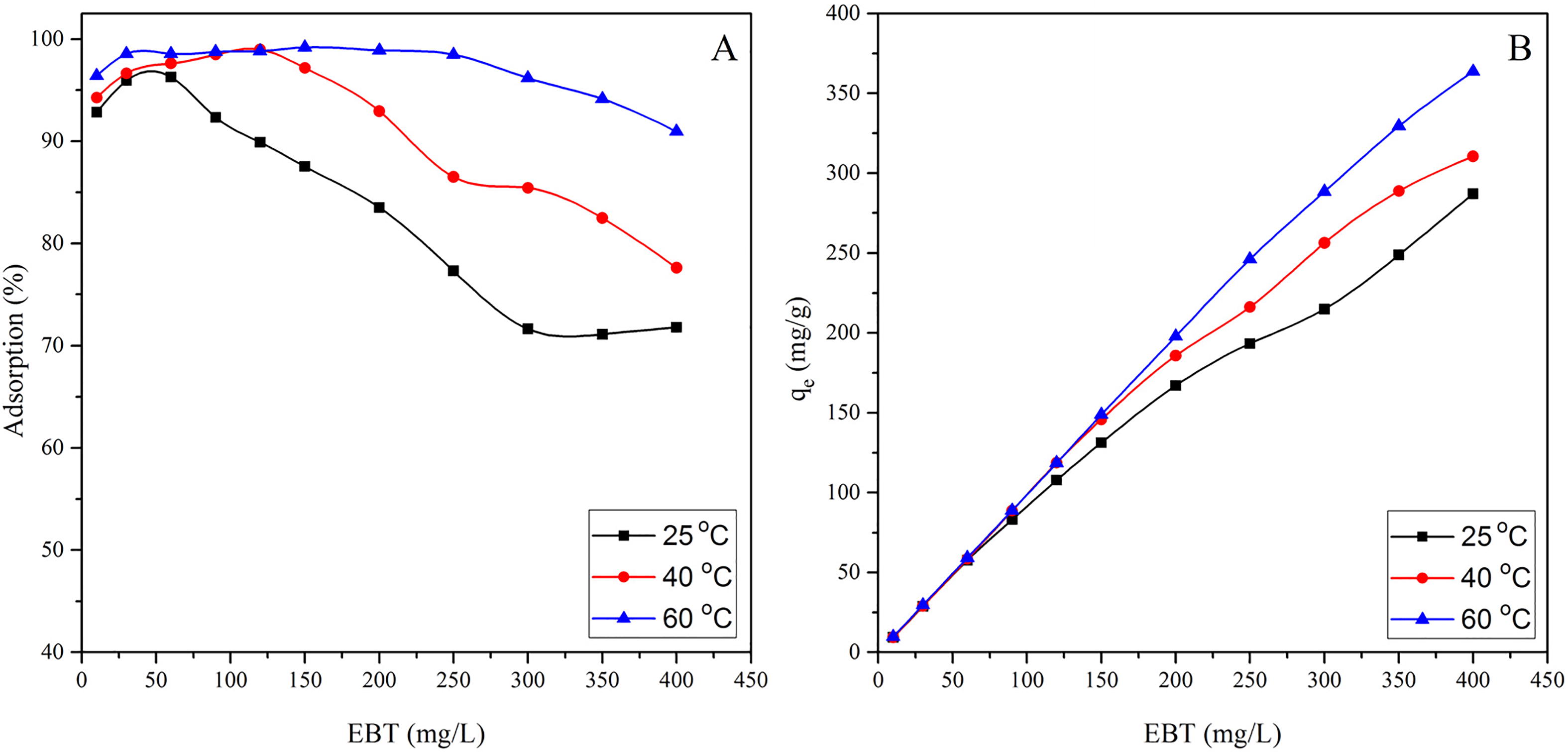
Fig. 4 Isotherm plots, Langmuir (A), Freundlich (B), Temkin (C) and Dubinin-Radushkevich model (D) for EBT adsorption onto MASTL, at pH = 2.0, m/V = 1.0 g/L, and t = 24 h.
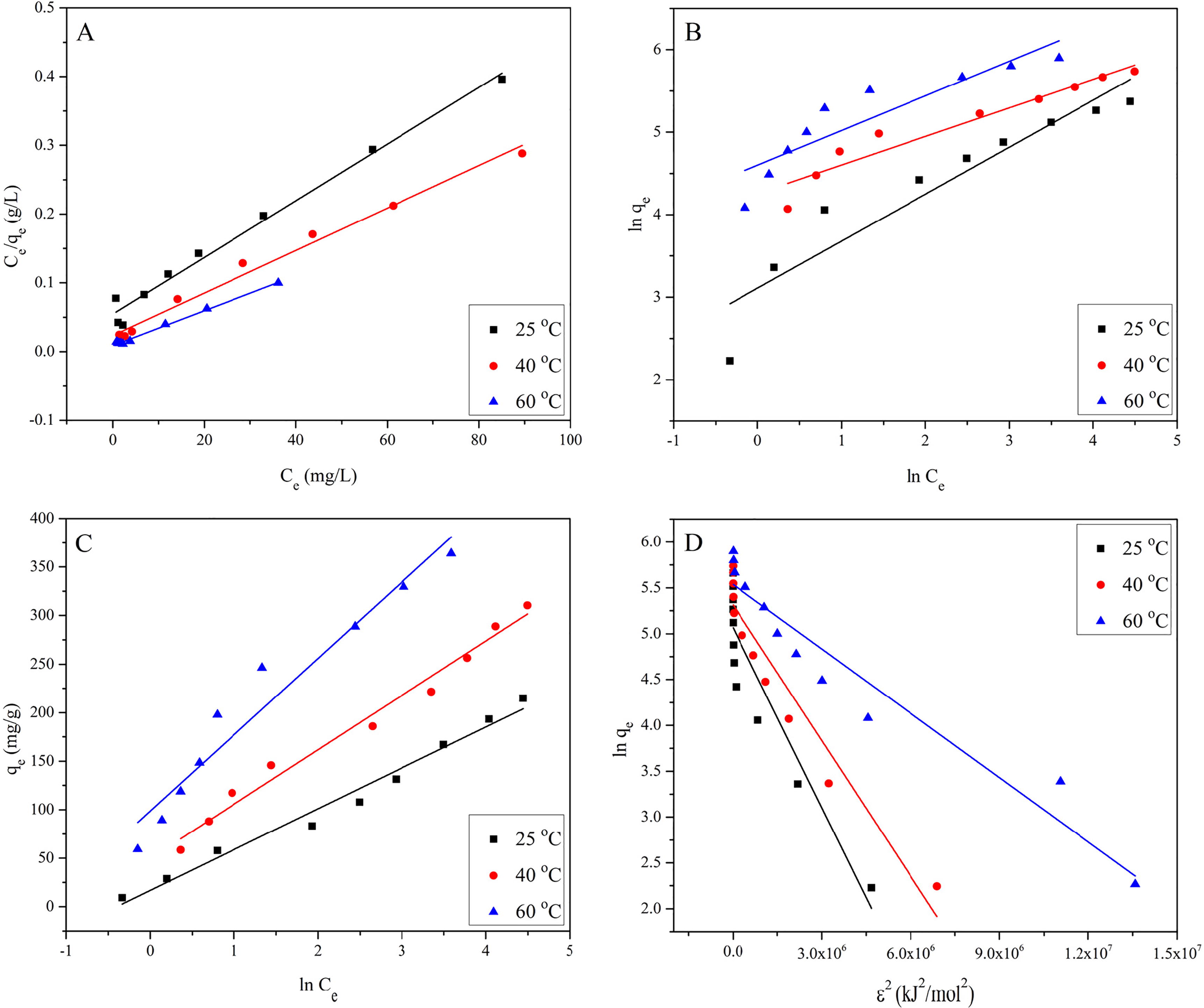
Table 1 Langmuir, Freundlich, Temkin and Dubinin-Radushkevich model constants and correlation coefficients for the adsorption of EBT onto MASTL.
Table 2 Comparison of equilibrium monolayer adsorption capacity of EBT on different adsorbents.
Table 3 Kinetic parameters for EBT adsorption onto MASTL. qe,exp = 57.70 and 57.99 mg/g at 25 and 40 °C respectively.
Fig. 6 The fitting of pseudo-first-order (A), pseudo-second-order (B), Intraparticle diffusion model (C) and Van't Hoff plot (D), for EBT adsorption onto MASTL, at pH = 2.0, m/V = 1.0 g/L, C[EBT]initial = 60 mg/L.
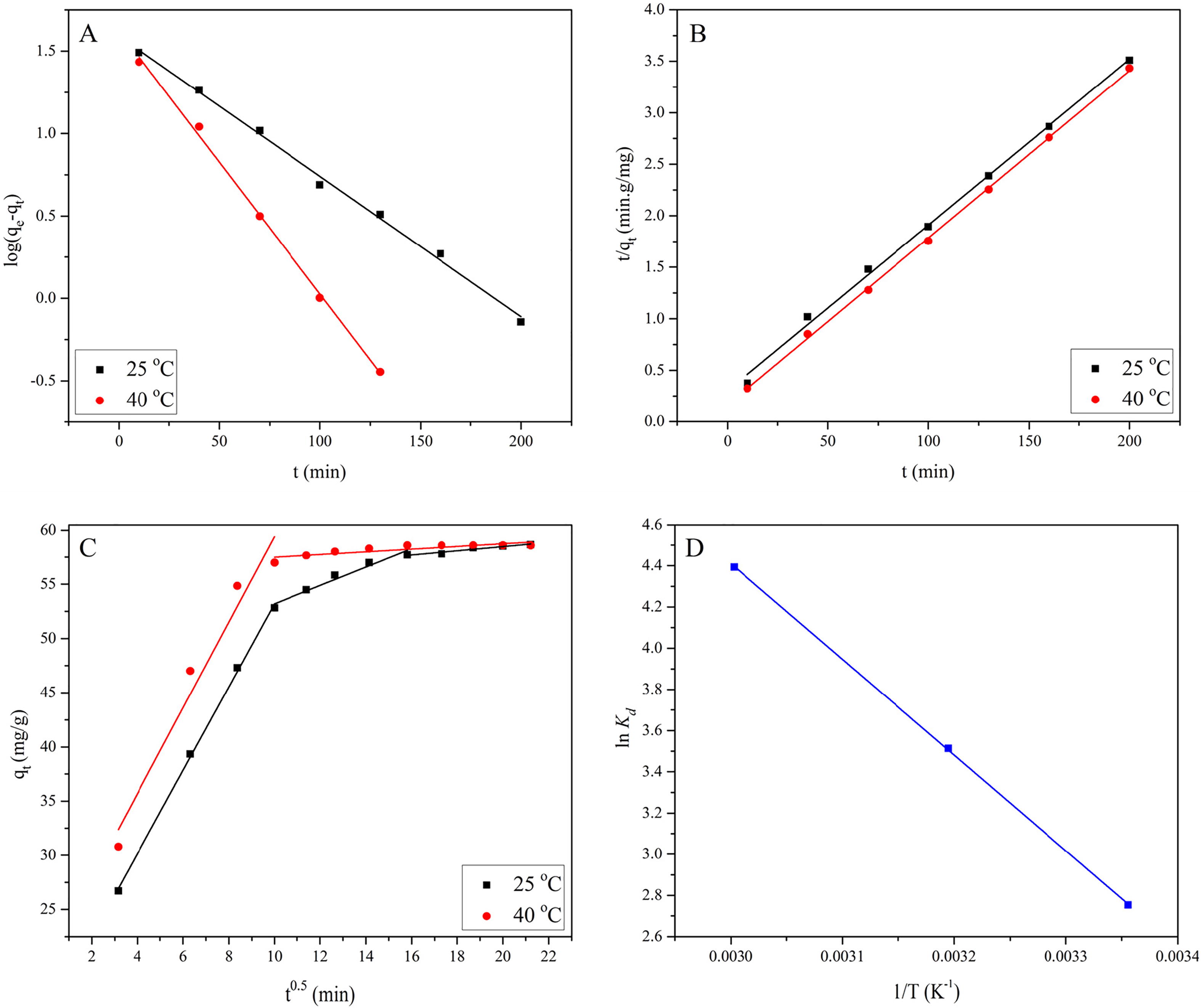
Table 4 Thermodynamic constants for EBT adsorption onto MASTL.
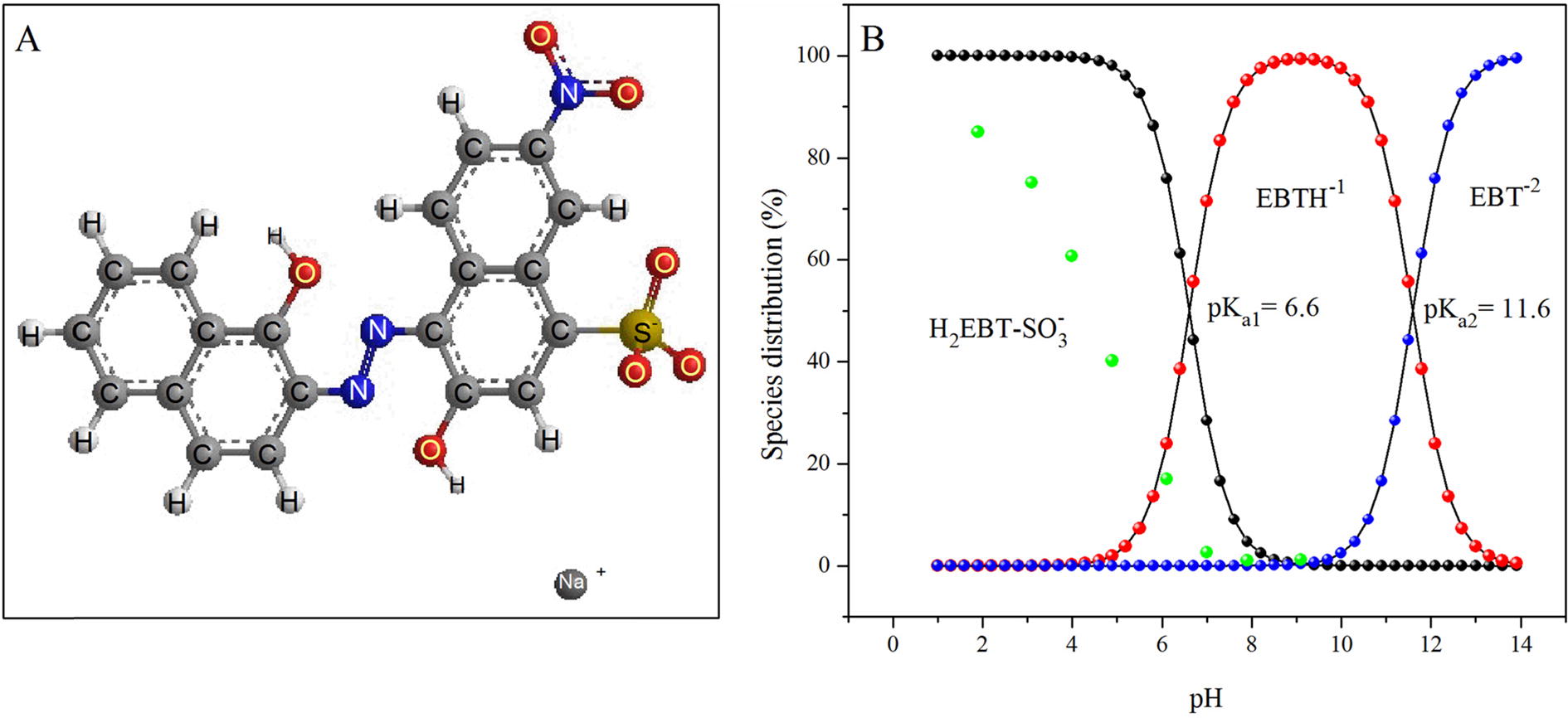
Fig. 7 Molecular structure of EBT (A) and distribution of various species of EBT and its percent adsorption (green balls) as a function of pH (B).