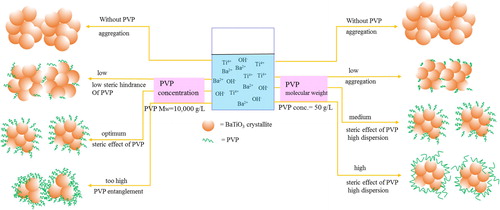
Figures & data
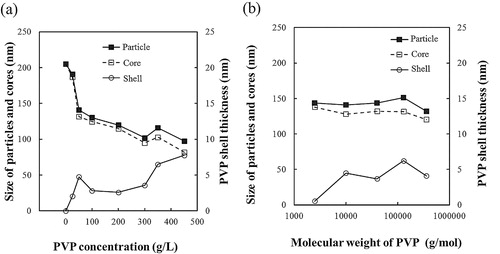
Table 1 Reaction conditions and results of BT–PVP synthesis using solutions with different PVP concentrations. The molecular weight of PVP was 10,000 g/mol.
Table 2 Reaction conditions and results of BT–PVP synthesis using solutions containing PVP with different molecular weights. The concentration of PVP was 50 g/L.
Fig. 1 (a) XRD patterns and (b) FE-SEM images of BT–PVP prepared at various PVP concentrations: (i) 0, (ii) 25, (iii) 100, and (iv) 450 g/L.
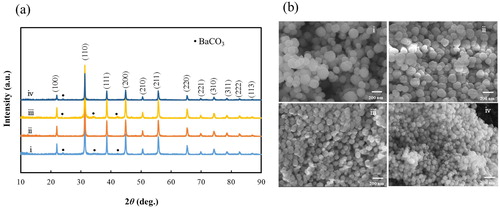
Fig. 2 (a) TEM image of BT–PVP prepared in 100 g/L PVP reaction solution. (b) TEM image of the BT–PVP particle marked by the red arrow point in (a). (c) HRTEM image of a part of the particle shown in (b). (d) Enlarged HRTEM image and (e) corresponding fast Fourier transform (FFT) pattern of the area enclosed by the red rectangle in (c).
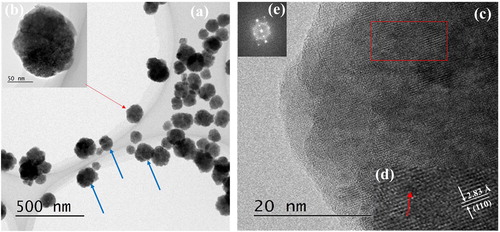
Fig. 3 (a) Effect of PVP concentration on the size of particles measured by FE-SEM and the size of crystallites estimated from the XRD peak corresponding to reflection from the (110) plane. (b) Effect of PVP concentration on the average number of crystallites in an agglomerated particle of BT–PVP.

Fig. 4 (a) DLS patterns of BT–PVP prepared at different concentrations of PVP: (i) 0, (ii) 25, (iii) 100, and (iv) 450 g/L. (b) Relationship between the particle size and PVP concentration. The particle size was measured FE-SEM and DLS respectively.
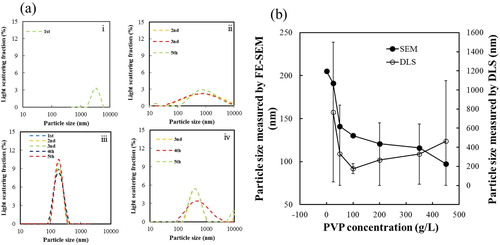
Fig. 5 (a) XRD patterns and (b) FE-SEM images of BT–PVP prepared from reaction solutions containing different molecular weight of PVP (i) 2500, (ii) 10,000, (iii) 130,000, and (iv) 360,000 g/mol.

Fig. 6 (a) Effect of PVP molecular weight on the size of particles measured by FE-SEM and the size of crystallites estimated from the XRD peak corresponding to reflection from the (110) plane. (b) Effect of PVP molecular weight on the average number of crystallites in an agglomerated particle of BT–PVP.
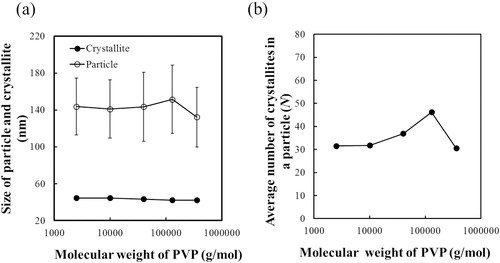
Fig. 7 (a) DLS patterns of BT–PVP prepared from solutions containing PVP with different molecular weight of PVP (i) 0, (ii)10,000, (iii)130,000, and (iv) 360,000 g/mol. (b) Relationship between particle size and molecular weight of PVP. The particle size was measured either by FE-SEM or DLS.
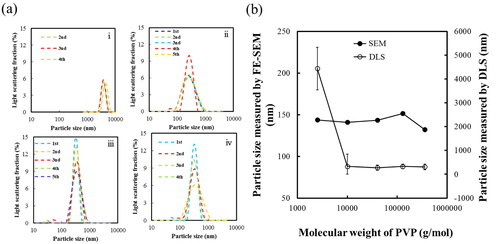
Fig. 8 (a) Relationship between pH and ζ-potential of three different types of nanoparticles: BT prepared without PVP; BT–PVP prepared from solution containing PVP with molecular weight of 2500 and 10,000 g/mol. FE-SEM images of an aqueous suspension containing 1 wt% nanoparticles of BT–PVP prepared with 10,000 g/mol PVP(b) and BT prepared without PVP(c).