Figures & data
Table 5 High to low spin transition energy and adsorption properties of NO interacting with regular (O2−) site and oxygen vacancy (Fs) site of the CdO (0 0 1) surface supported Pd atoms at the low (2)- and high spin states (4).
Table 6 High to low spin transition energy and adsorption properties of NO interacting with regular (O2−) site and oxygen vacancy (Fs) site of the CdO (0 0 1) surface supported Pd2 with parallel configuration of high- and low-spin states.
Table 7 High to low spin transition energy and adsorption properties of NO interacting with regular (O2−) site and oxygen vacancy (Fs) site of the CdO (0 0 1) surface supported Pd2 with perpendicular configuration of high- and low-spin states.
Fig. 1 Schematic representation of the embedded cluster Cd9O14 Red spheres: O2−; yellow spheres: Cd2+.
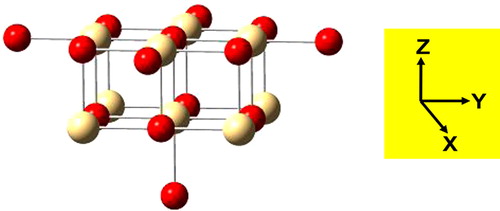
Fig. 2 Schematic representation of the highest occupied molecular orbitals (HOMOs) of Pd atoms at (a) low spin state and (b) high spin state that deposited on CdO(O2−) and CdO(Fs) sites using the embedded cluster model.
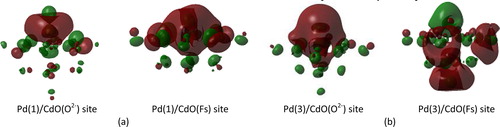
Table 1 Main energetic, geometric and electronic population parameters for Pd atoms in the high (3)- and low (1)-spin states that deposited on a regular oxygen site “CdO (O2−)” and on Fs centre “CdO (Fs)” energies are expressed in eV, distances in Å and charges in electron units.
Table 2 High to low spin transition energy calculated from relation (2) of supported Pd atoms. A positive sign indicates that the ground state is provided by the low-spin coupling. ΔdH−L are the change in the equilibrium distances of supported Pd atoms from high- to low-spin state. A positive value indicates that d is larger in the high-spin state. Energies are expressed in eV, the corresponding equilibrium distances (d) in (Å), H: high spin; L: low spin.
Table 3 Geometrical parameters, binding, nucleation, trapping energies and charges for the adsorption of Pd2 dimers with various spin multiplicity at regular (O2−) site and defect centre (Fs) site of the CdO (0 0 1) surface. Energies are expressed in eV, the corresponding equilibrium distances (d) in (Å), and charges in electron units.
Fig. 3 Schematic representation of (a) parallel Pd2 particles deposited on regular CdO (O2−) site; (b) parallel Pd2 particles deposited on defective CdO (Fs) site; (c) NO adsorbed perpendicular on Pd atom and (d) Pd2 deposited on regular CdO(O2−) site
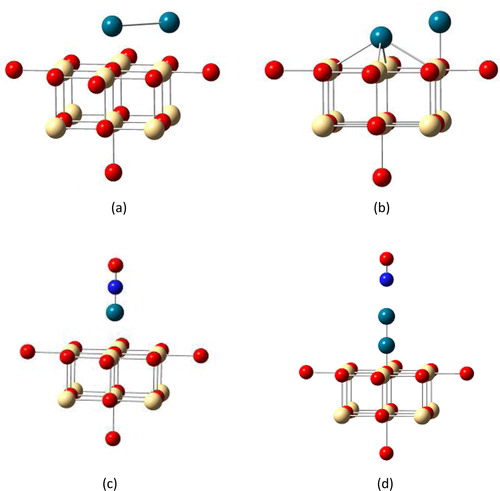
Table 4 High to low spin transition energy calculated from relation (2) of supported Pd2 dimer. A positive sign indicates that the ground state is provided by the low-spin coupling.
and
are the change in the equilibrium distance of each Pd atoms to the surface;
is the change in charges at the dimers that supported to the regular (O2−) site and oxygen vacancy (Fs) site at CdO (0 0 1) with parallel and perpendicular configurations going from high- to low-spin state. A positive value indicates that d is larger in the high-spin state. Energies are expressed in eV, the corresponding equilibrium distances (d) in (Å), and charges in electron units H: high spin. L: low spin.
Fig. 4 Schematic representation of the HOMOs of NO/Pd/CdO, Pd2/CdO, NO/Pd2/CdO complexes deposited on CdO (O2−) and CdO (Fs) sites using the embedded cluster model. Where low spin state for Pd and Pd2 is (1) and high spin is (3).
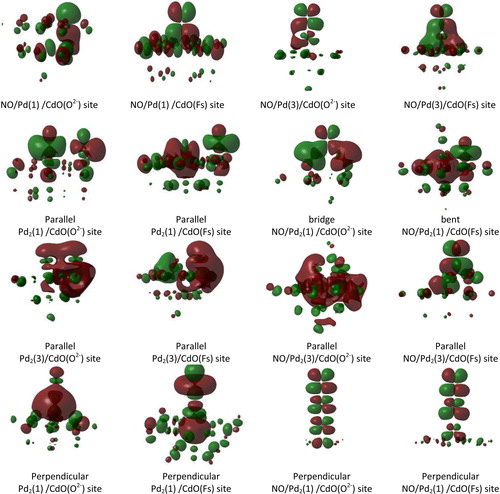
Table 8 The electronic configurations of Pd from NBO in Pd/CdO, Pd2/CdO, NO/Pd/CdO, NO/Pd2/CdO complexes at (O2−) and (Fs) sites in their low and high spin states.
Fig. 5 Total charge–density contours in the (0 0 1) plane of CdO clean surface and the adsorption of NO on Pd- and Pd2-supported at CdO (O2−) and CaO (Fs).
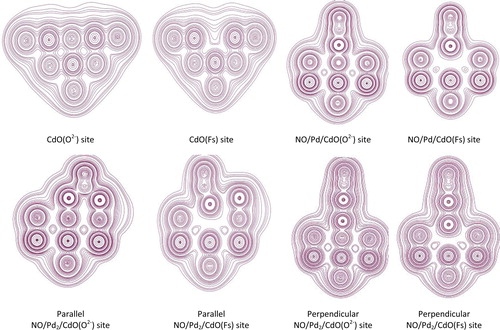
Table 9 Tops of the valance bands (V.B.), and the bottoms of conductions bands (C.B.), of defect-free surfaces of CdO crystals. As well as the highest occupied molecular orbitals (HOMOs) and the lowest unoccupied molecular orbitals (LUMOs) of Pd/CdO, Pd2/CdO, NO/Pd/CdO, NO/Pd2/CdO complexes at the defect-containing surfaces in their low and high spin states.
Fig. 6 Frontier orbitals of regular surfaces Cd9O14, free NO molecule, Pd/Cd9O14, Pd2/Cd9O14, NO/Pd/Cd9O14 and NO/Pd2/Cd9O14 with parallel and perpendicular modes at their high and low spin states.
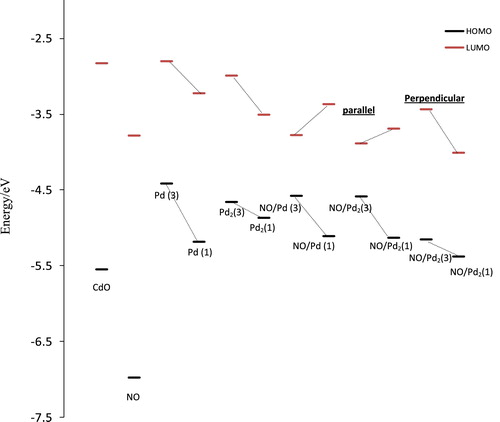
Fig. 7 Density of states (DOS), HOMOs and LUMOs for CdO(O2−), CdO(Fs), NO/Pd/CdO(O2−) and NO/Pd/CdO(Fs) complexes. Vertical lines: Fermi levels.
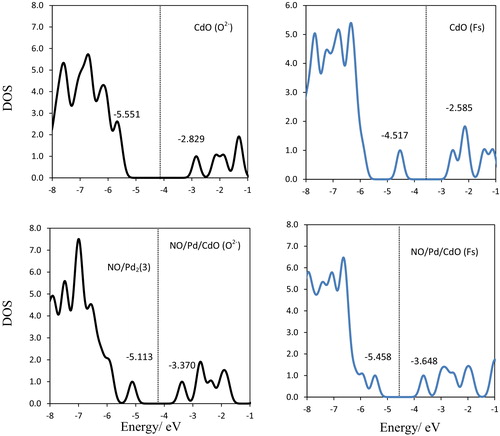
Fig. 8 Density of states (DOS), HOMOs and LUMOs for NO/Pd2/CdO(O2−) and NO/Pd2/CdO(Fs) complexes with parallel and perpendicular modes. Vertical lines: Fermi levels.
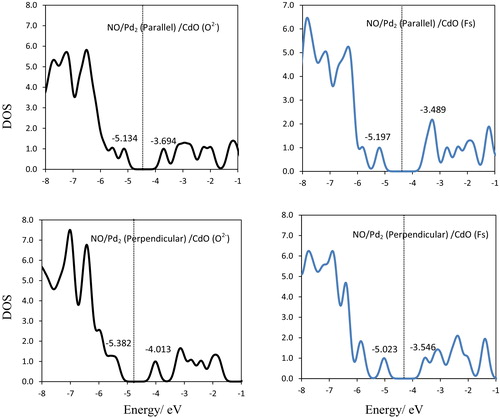
Table 10 Spin contamination correlated with energetic properties of ON/Pd/CdO and ON/Pd2/CdO with parallel configuration complexes in their low and high spin states.
Table 11 Spin states and interaction energies of ON.Pdx.CdO complexes (parallel mode) at O2− and Fs sites, pairwise components, and non additivity terms. All energies are given in eV.
