Figures & data
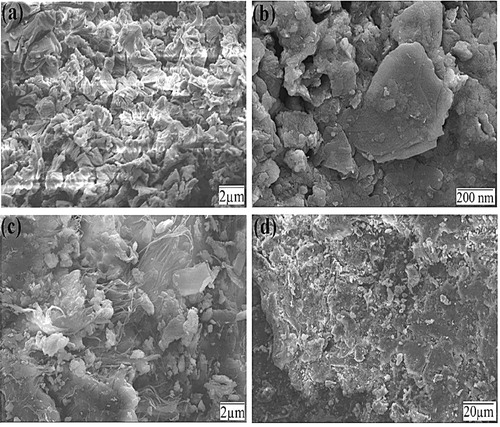
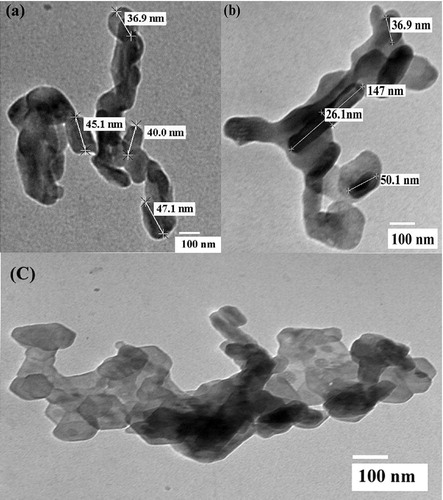
Fig. 3 (a) UV–vis absorbance spectra of ZnWO4/CaO composites. (b) X-ray diffraction pattern of pure CaO and ZnWO4/CaO composites. (c, d) EDX pattern of pure CaO and ZnWO4/CaO composites.
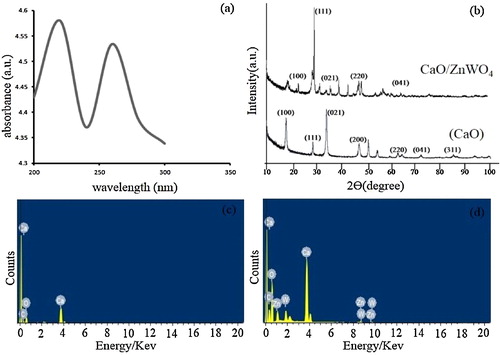
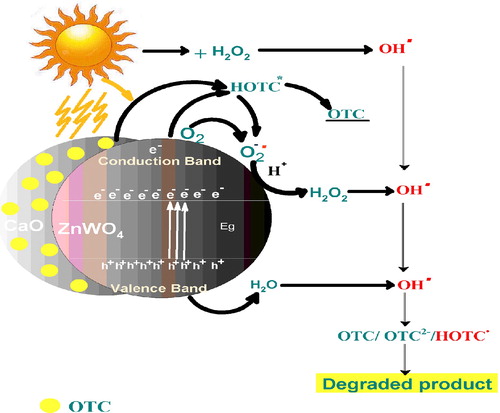
Fig. 5 (a, b) Time profile of OTC degradation in the H2O2/CaO/ZnWO4 system under different reaction conditions (a) solar and (b) in the dark. (c) Time profile for adsorptional photocatalytic degradation of OTC. (d) The plot of log (absorbance) vs. time. Experimental conditions: [OTC] = 1 × 10−4 M, [H2O2] = 1 × 10−4 M, Catalyst dosage = 50 mg/50 ml, pH 5, temperature = 30 ± 0.3 °C and time = 70 min.
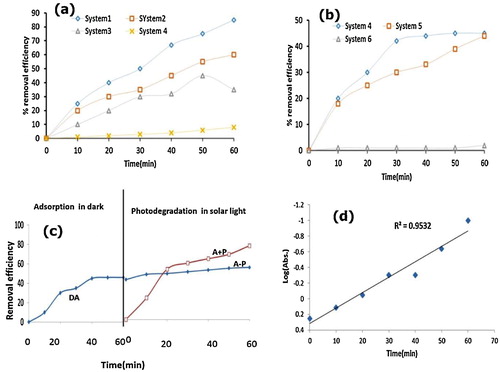
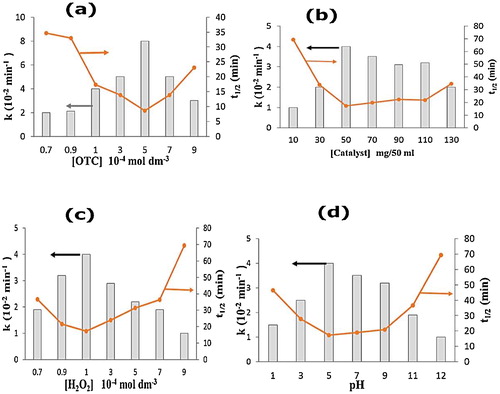
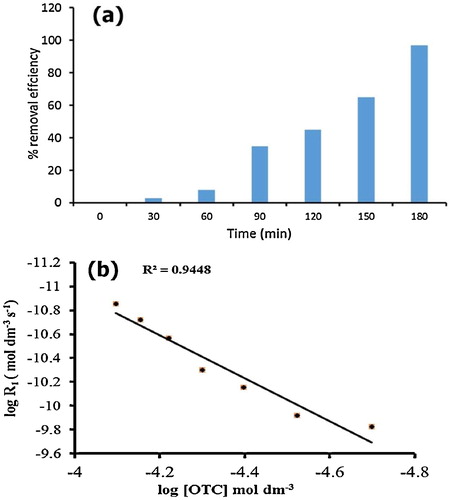
Fig. 6 (a–d) Effect of different reaction parameters, OTC (a), catalyst (b), H2O2 (c), pH (d) on OTC removal. Reaction conditions [OTC] = 1 × 10−4 M, catalyst dosage = 50 mg/50 ml, [H2O2] = 1 × 10−4 M, pH 5, temperature = 30 ± 0.3 °C, reaction time = 60 min and solar light intensity = 35 × 103 ± 1000 lx.