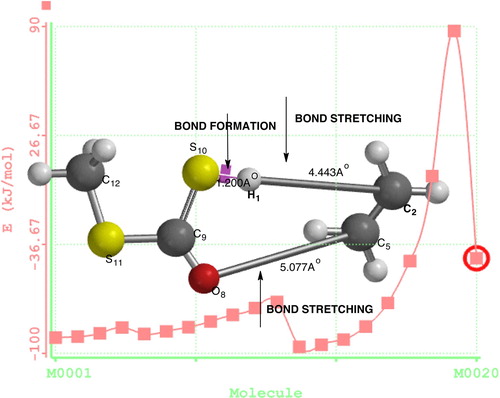
Figures & data
Table 2 Selected bond angles in degrees for the pyrolysis of O-ethyl, S-methyl xanthate (dithiocarbonates).
Table 3 Selected dihedral angles in degrees for the pyrolysis of O-ethyl S-methyl xanthate (dithiocarbonate).
Table 1 Selected bond lengths (Å) for the pyrolysis of O-ethyl, S-methyl xanthate (dithiocarbonate). Δd = (TS − GS).
Table 4 Selected atomic charges (Mulliken). Δq = (TS − GS) for the pyrolysis of O-ethyl-S-methyl xanthate.
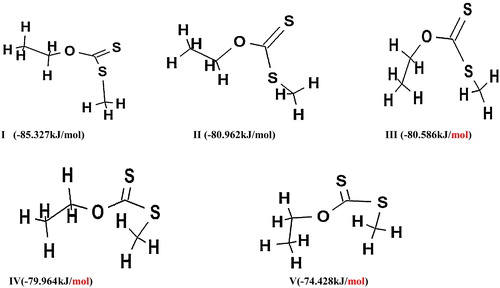
Table 5 Heat of formation for the pyrolysis of O-ethyl, S-methyl xanthate (dithiocarbonates).
Table 6 Arrhenius parameters for O-ethyl S-methyl xanthate (dithiocarbonates).
Table 7 Variation of rate of reaction (S−1) with temperature (K).
Table 8 B3LYP/6-31G* calculated Wiberg bond indices, Bi, for the reactants, transition states and products: percentage of evolution through the chemical process of the bond indices at the transition states %Ev, degrees of advancement of the transition states δBav, and absolute synchronicities Sy for pathway A for the pyrolysis of O-ethyl, S-methyl dithiocarbonates.