Figures & data
Table 1 Densities and apparent molar volumes of solutions of copper sulfate in aqueous ethanol at different temperatures.
Table 2 Limiting apparent molar volumes (Vϕ°) and experimental slopes (BV) of copper sulfate in water, and aqueous ethanol solutions at different temperatures.
Fig. 1 CuSO4 partial molar volumes of transfer from H2O to EtOH–H2O solutions at different temperatures.
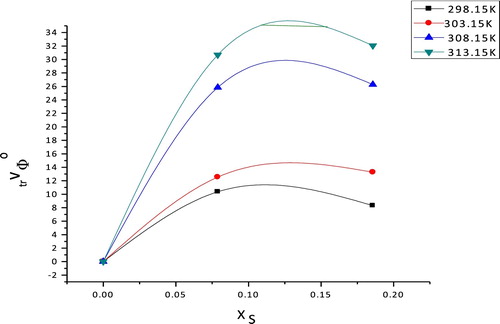
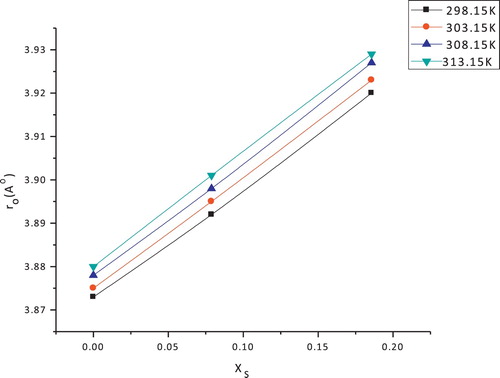
Table 3 Molar volume (V), Van der Waals volume (Vw), electrostriction volume (Ve) in (Cm3 mol−1) and solvated radii (ro) of copper sulfate in (EtOH–H2O) mixed solvents at different temperatures.
Table 4 Limiting molar conductance (Λ°m) for copper sulfate in aqueous ethanol from T = 298.15–313.15 K.
Table 5 Association constant KA and changes in Gibb’s energy (ΔG°A) for used salt in water + EtOH at different temperatures.
Fig. 3 Plots of Walden product Λm°ηo/(S cm2 mol−1 kg m−1 s−1) against volume fraction φv for copper sulfate in water + ethanol from T = 298.15–313.15 K.
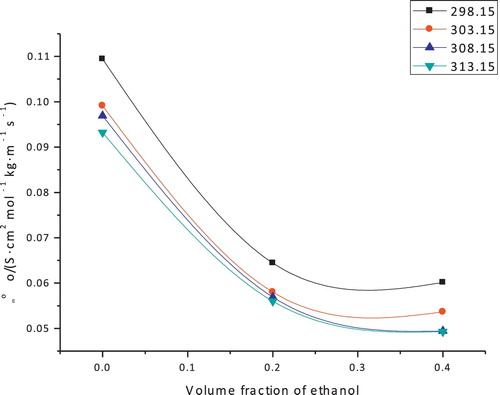
Table 6 Entropy change of association (ΔS°A) and enthalpy change of association (ΔH°A) for copper sulfate in used solvents at different temperature.
Table 7 Activation energy (Ea) for copper sulfate in EtOH–H2O solvents.