Figures & data
Fig. 1 Variation of ultrasonic velocity, U vs Conc. of lanthanum(III) chloride in water and sorbitol-water mixture at temperature 303.15 K.
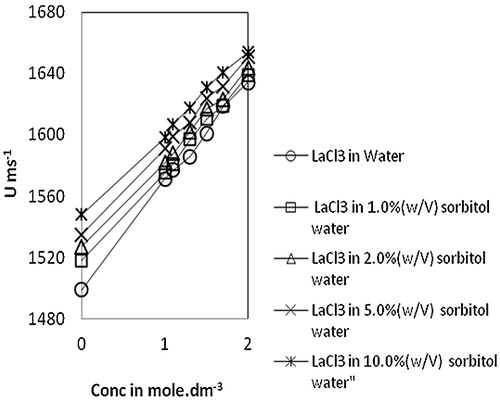
Table 1 Experimentally determined ultrasonic velocity, U, density, ρ, viscosity η, and calculated values of apparent molar volume, ϕv, Relative association, RA, solvation number, Sn , and relaxation time, τ of lanthanum(III) chloride in water and different proportions of sorbitol-water mixture at 303.15 K.
Fig. 2 Variation of isentropic compresiibility, βs vs Conc. of lanthanum(III) chloride in water and sorbitol-water mixture at temperature 303.15 K.
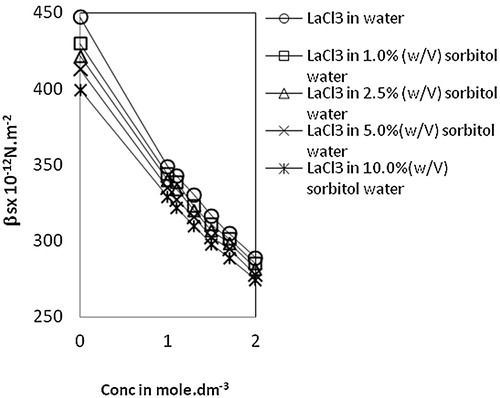
Fig. 3 Variation of intermolecular free length, Lf vs Conc. of lanthanum(III) chloride in water and sorbitol-water mixture at temperature 303.15 K.
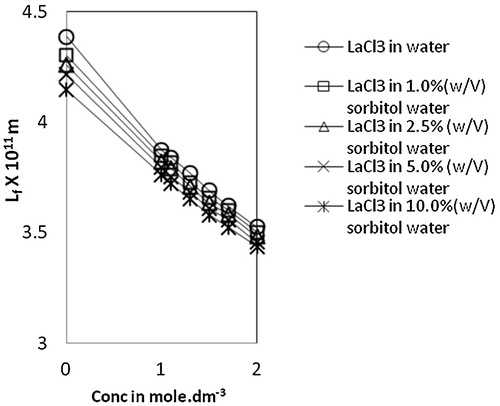
Fig. 4 Variation of acoustic impedance, Z vs Conc. of lanthanum(III) chloride in water and sorbitol-water mixture at temperature 303.15 K.
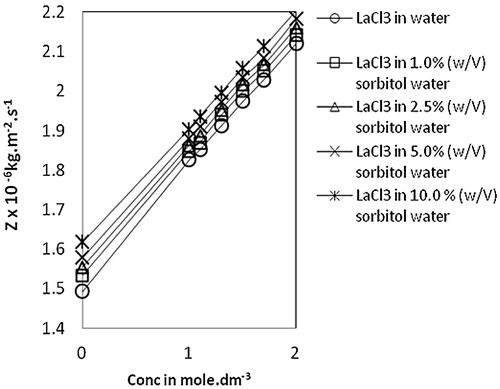
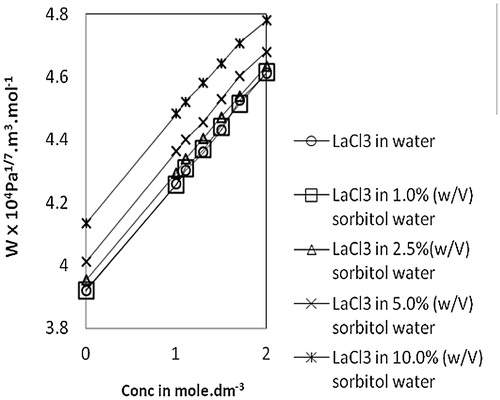
Table 2 Thermodynamic and acoustic parameters of lanthanum(III) chloride in water and different proportions of sorbitol-water mixture at 303.15 K.
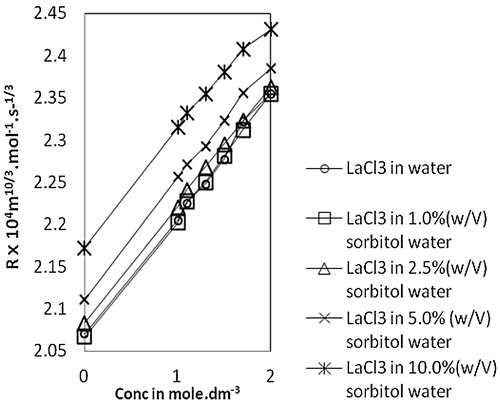
Fig. 5 Variation of molar volume, R vs Conc. of lanthanum(III) chloride in water and sorbitol-water mixture at temperature 303.15 K.