Figures & data
Figure 1 Schematic showing the process of enterovirus uncoating. The mature virion (left) comprises 60 copies each of VP1, VP2, VP3 and VP4, and agenomic RNA. The virion is captured by its cognate receptor on the target cell surface and then internalized. The Ig-like domain in PVR, CAR and ICAM-1 binds to the canyon of the virus and induces a conformational change. The A-particle (middle) comprises 60 copies each of VP1, VP2 and VP3, together with the genomic RNA. The A-particle increases in diameter by approximately 4% and has a large hole near the two- and three-fold axes. The N-terminus of VP1 is externalized and anchors the virus to the membrane, where extruded VP4s associate to form a channel through the membrane. The viral RNA is then released from the hole close to the two-fold axis and enters the cell cytoplasm. Minor group human rhinoviruses bind to LDLR family members and the conformational change of the virionis induced by the low endosomal pH. The resulting empty capsid (right) comprises 60 copies each of VP1, VP2 and VP3. CAR, coxsackie-adenovirus receptor; ICAM-1, intercellular adhesion molecule-1; LDLR, low-density lipoprotein receptor.
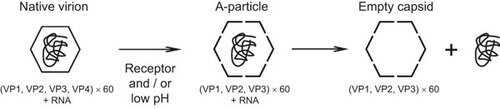
Table 1 EV71 receptors and their functions
Figure 2 EV71 infection mediated by SCARB2 and other receptors. SCARB2 delivers β-GC from the ER to the lysosomes under physiological conditions. SCARB2 is abundant in the lysosomal and endosomal compartments, and it also shuttles to the plasma membrane where it encounters EV71. After binding the virus on the cell surface, the virus–receptor complex is internalized via the clathrin-mediated endocytosis pathway. In the endosome or lysosome, where the pH is low, the virus initiates a conformational change that leads to uncoating. PSGL-1 can bind EV71 and internalize via caveolin-mediated endocytosis, but PSGL-1 cannot initiate uncoating. Anx2, heparan sulfate, and sialylated glycans can also bind EV71. However, the mechanism of internalization and uncoating is unknown. They may deliver EV71 to SCARB2 or they may establish infections via their own mechanisms. β-GC, β-glucocerebrosidase; ER, endoplasmic reticulum.

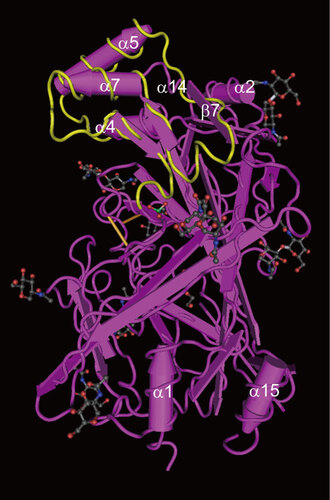
Figure 3 Crystal structure of SCARB2. The crystal structure of the SCARB2 ectodomain (PDB: 4F7B) was determined by X-ray diffraction. The important amino acid region responsible for binding to EV71 (amino acids 142–204)Citation56 is shown in yellow. Arrows represent β-strands and tubes represent α-helices. Black, red and blue sticks and balls represent the carbohydrate chains.