Figures & data
Table 1 Primers used in the study
Figure 1 Serologic reactivity of recombinant EF-Tu (rEF-Tu) in infected mice and Lyme disease patients. (A) SDS-PAGE showing purified recombinant EF-Tu protein. Predicted size and observed size for B. burgdorferi EF-Tu is 43 kDa and 47 kDa, respectively. (B) Immunoblotting of rEF-Tu using sera collected from C3H/HeN mice infected with B. burgdorferi strain 5A4NP1. Mouse sera used were collected at 2, 3, 4, 6 weeks of post-infection (1:600 dilution). *, HS stands for hyperimmune sera from mice immunized with rEF-Tu (αEF-Tu, 1:10000 dilution). ^, N stands for sera from naïve mice (1:600 dilution). (C) Representative immunoblotting results showing serologic reactivity of rEF-Tu in Lyme disease patients. Strips C-K are rEF-Tu probed with sera from ten different Lyme disease patients randomly selected from the CDC Lyme disease patient sera panel (1:100 dilution).
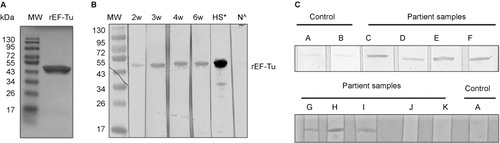
Table 2 Seroreactivity of Lyme disease patient serum against recombinant EF-Tu protein
Figure 2 Active immunization with rEF-Tu does not protect mice from B. burgdorferi, and anti-EF-Tu antibody does not affect spirochetal survival and migration in in the tick vector. (A) Quantitation of spirochete load in mice. Tibio-tarsal joints from mice immunized with rEF-Tu or non-immunized mice (labeled as ‘Adjuvant’) were subjected to qPCR analyses (n = 4 mice/group). The B. burgdorferi flaB gene was used as target, and the levels were normalized with the mouse Nidogen gene. Data denote the mean ± the standard error (SEM) from two separate experiments. Differences between mice immunized with rEF-Tu and controls were analyzed using a Student t test (P value ≤ 0.05). (B) qPCR analyses of spirochete load in larvae fed on B. burgdorferi-infected mice that were either immunized with EF-Tu (which contains high levels of anti-EF-Tu antibodies) or non-immunized. Bars represent the mean ± SEM from 18 fed larval ticks collected from immunized mice and 9 fed larval ticks collected from controls (n = 2 mice/group). Data are representative of two separate experiments. Copies of the flaB genes were normalized with tick actin gene.
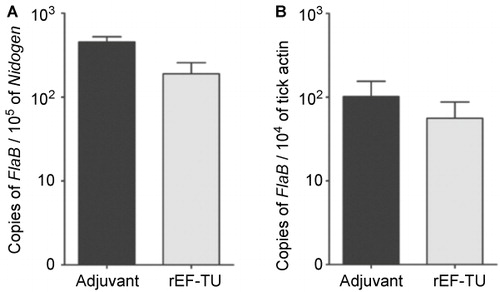
Table 3 Culture results of mice immunized with rEF-TU. Culture results expressed as number of mice infected/total number of mice tested
Figure 3 B. burgdorferi EF-Tu is not surface-localized. (A) IFAs of fixed (top panels) or unfixed (bottom panels) wild-type B. burgdorferi strain B31-A3 probed with monoclonal antibodies directed against outer surface protein OspA, periplasmic protein FlaB, or EF-Tu as primary antibodies, and FITC-conjugated goat anti-mouse antibody as secondary antibodies. (B) Proteinase K protection assay of wild-type B. burgdorferi. Intact cells were incubated with proteinase K (200 μg/mL) for 1 h. Washed cells were then subjected immunoblotting using a mixture of antibodies against OspA, FlaB, and EF-Tu. (C) Proteinase K protection assay of B. burgdorferi overexpressing EF-Tu. Intact cells were incubated with proteinase K (25, 50, 100, or 200 μg/mL) for 1 h and then subjected to SDS-PAGE (left panel and black arrow indicates the observed size of B. burgdorferi EF-Tu) or immunoblotting using a mixture of antibodies against OspA, FlaB, and EF-Tu (right panel). Data are representative of four separate experiments.
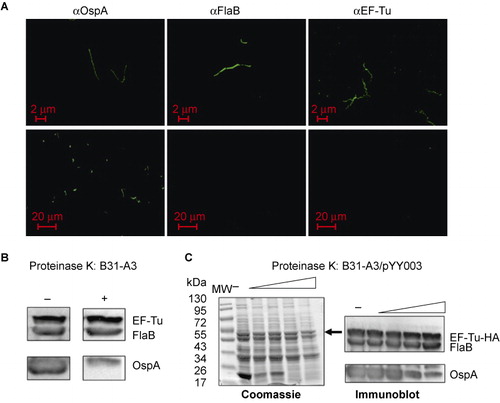
Figure 4 Construction of the B. burgdorferi strain overexpressing EF-Tu. (A) Diagram of the shuttle vector pYY003 carrying a tuf gene under the control of a strong and constitutive flaB promoter. (B) Coomassie-stained gel showing overexpression of EF-Tu. Lane 1, wild-type strain B31-A3; lane 2, B31-A3/PflaB-tuf-HA. The band corresponding to EF-Tu was indicated by arrow. (C) Confirmation of over-production of HA-tagged EF-Tu by immunoblotting using either HA monoclonal antibody or antibody against FlaB (loading control). Lane 1, wild-type strain B31-A3; lane 2, B31-A3/PflaB-tuf-HA.
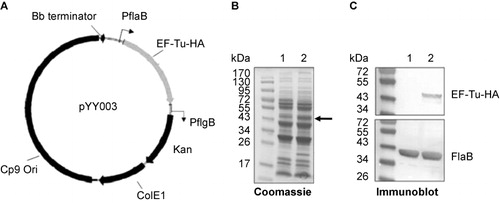
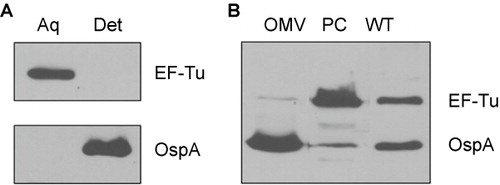
Figure 5 B. burgdorferi EF-Tu is a soluble protein associated with the PC fractions. (A) Immunoblot analyses of detergent-enriched (Det) and aqueous-enriched (Aq) phases of B. burgdorferi with antibodies against EF-Tu or OspA. (B) Immunoblot analyses of PC fraction and outer membrane vesicles (OMVs) of B. burgdorferi separated by sucrose density gradient centrifugation. Whole cell lysates of wild-type B. burgdorferi (WT) were used for comparison. Equal amounts of proteins were loaded and immunoblotted with antibodies directed against EF-Tu or OspA.