Figures & data
Figure 1 Kinetics of the serological response in a MERS-CoV-infected patient as determined by ELISAs for various recombinant antigens and the ppNT. Sera sampled on days 2, 3, 4, 5, 6, 8, 9, 10, 12, 13, 15, 18, 20, 27 and 28 after admission to the hospital were retrospectively analyzed. (A) Longitudinal profiles of IgG antibodies against MERS-CoV, S, S1, NTD, RBD and NP in the patient who was the first to import MERS-CoV into China; sera were determined by 1: 80 dilution in all ELISAs except by 1:101 dilution in S1 ELISA. (B) Longitudinal profiles of IgM antibodies against MERS-CoV, S, NTD, RBD and NP; sera were determined by 1:80 dilutioin in all ELISAs. (C) Longitudinal profiles of NAb against MERS-CoV. enzyme-linked immunosorbent assay, ELISA; immunoglobulin G/M, IgG/M; Middle East respiratory syndrome coronavirus, MERS-CoV; neutralizing antibody, NAb; nucleoprotein, NP; N-terminal domain, NTD; optical density, OD; pseudovirus particle-based neutralization test, ppNT; receptor-binding domain, RBD; recombinant MERS-CoV full-length spike protein, S.
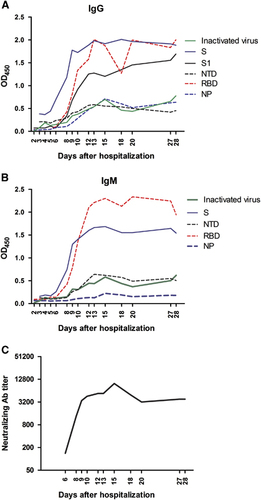
Figure 2 Sensitivities of ELISAs based on different viral antigens. ELISA plates were coated with inactivated MERS-CoV particles (A) or purified recombinant S (B), S1 (C), NTD (D), RBD (E) or NP (F) protein. Serum samples from the patient with MERS-CoV collected on days 2 (sample 1), 15 (sample 2) and 28 (sample 3) after admission to the hospital were serially diluted and dispensed into the wells of an ELISA plate. HRP-labeled goat anti-human IgG (left) and IgM (right) were used as the secondary antibody, with 3,3',5,5'-tetramethylbenzidine (TMB) as the substrate. The results are expressed as the absorbance reading at 450 nm. As the negative control had very poor ELISA responses, the results are not shown to avoid interference with the target profiles. enzyme-linked immunosorbent assay, ELISA; horseradish peroxidase, HRP; immunoglobulin G/M, IgG/M; Middle East respiratory syndrome coronavirus, MERS-CoV; nucleoprotein, NP; N-terminal domain, NTD; optical density, OD; receptor-binding domain, RBD; recombinant MERS-CoV full-length spike protein, S.
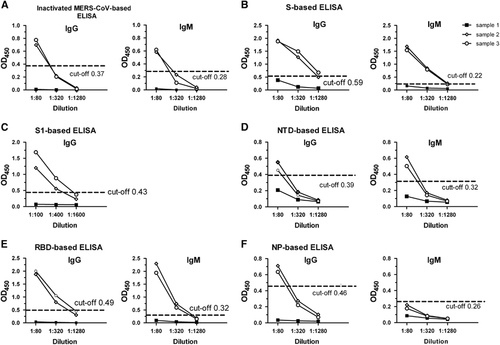
Figure 3 Correlations among the inactivated virus-based and other S or N protein-based ELISA results. The absorbance readings of the S (A), S1 (B), NTD (C), RBD (D) and NP (E) based IgG ELISAs (OD450 values) were plotted against that of the virus-based IgG ELISA (OD450 values) (data are those from ). ***P<0.001. Enzyme-linked immunosorbent assay, ELISA; immunoglobulin G, IgG; Middle East respiratory syndrome coronavirus, MERS-CoV; nucleoprotein, NP; N-terminal domain, NTD; optical density, OD; receptor-binding domain, RBD; recombinant MERS-CoV full-length spike protein, S.
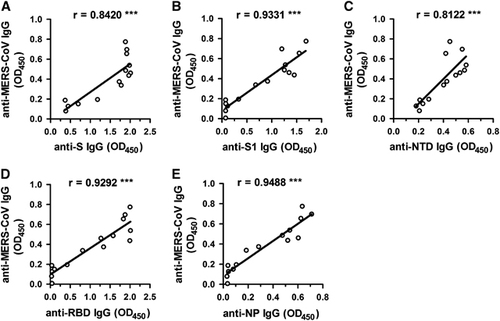
Figure 4 Correlation of the Euroimmune EIA Kit for detecting anti-S1 IgG antibody of MERS-CoV (OD450 values) with other recombinant protein-based IgG ELISAs (OD450 values) used in this study. The absorbance readings of the S (A), NTD (B) and RBD (C) based IgG ELISAs (OD450 values) were plotted against those of the S1-based IgG ELISA (OD450 values) (data are those from ). ***P<0.001. Enzyme-linked immunosorbent assay, ELISA; immunoglobulin G, IgG; Middle East respiratory syndrome coronavirus, MERS-CoV; nucleoprotein, NP; N-terminal domain, NTD; optical density, OD; receptor-binding domain, RBD; recombinant MERS-CoV full-length spike protein, S.
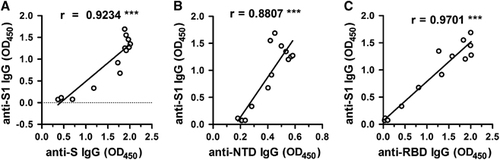
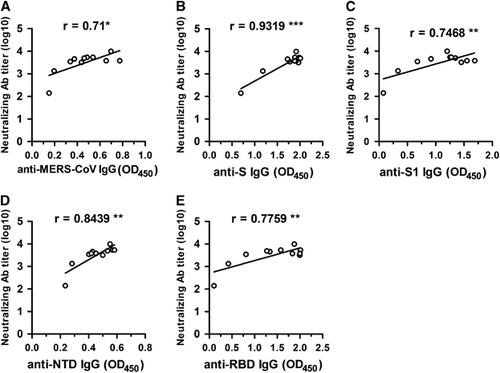
Figure 5 Correlations among the ELISAs (IgG) (OD450 values) and the MERS-CoV ppNT assay (neutralizing antibody titer) used in this study. The absorbance readings of the inactivated MERS-CoV (A), S (B), S1 (C), NTD (D) and RBD (E) based IgG ELISAs (OD450 values) (data are those from ) were plotted against neutralizing antibody titers (data are those from ). *P<0.05; **P<0.01; ***P<0.001. Antibody, Ab; enzyme-linked immunosorbent assay, ELISA; immunoglobulin G, IgG; Middle East respiratory syndrome coronavirus, MERS-CoV; nucleoprotein, NP; N-terminal domain, NTD; optical density, OD; pseudovirus particle-based neutralization test, ppNT; receptor-binding domain, RBD; recombinant MERS-CoV full-length spike protein, S.