Figures & data
a Schematic diagrams of the constructs made in this study. TRP2-L and TRP2-R, the up-stream and down-stream parts of the TRP region, used for homologous recombination; PAOX1, AOX1 promoter; CYC1 TT, CYC1 transcription termination region; ADE2, expression cassette encoding phosphoribosylaminoimidazole carboxylase, used as the selection marker. b ELISA analysis of cell lysates from pEV-D68-003-transformed yeast clones. A polyclonal antibody against inactivated EV-D68 was used for detection. Cell lysate from a yeast clone transformed with the empty vector pPink-HC was used as the negative control (ctr). Data are mean ± SD of triplicate wells. c Western blotting analysis with an anti-VP3 polyclonal antibody. Lane M, marker; lane 1, empty vector-transformed yeast; lane 2, pEV-D68-003-transformed yeast; lane 3, inactivated EV-D68 virus
a–b Sucrose gradient analyses. Lysates from pEV-D68-003-transformed yeast were subjected to 10–50% sucrose gradient sedimentation. Twelve fractions were collected from top to the bottom and analyzed by (a) Western blotting with anti-VP0, anti-VP1, or anti-VP3 polyclonal antibodies, respectively, and (b) ELISA with a polyclonal antibody against inactivated EV-D68. c Electron microscopy of EV-D68 VLPs. Bar = 100 nm
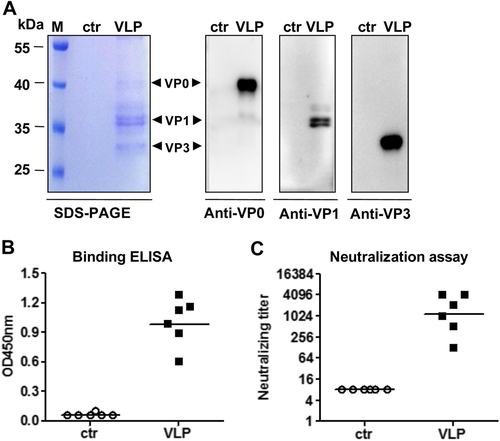
a SDS-PAGE and Western blotting analysis of the purified antigens. Lane M, protein marker; ctr, the control antigen prepared from empty vector-transformed yeast; VLP, purified EV-D68 VLPs. b ELISA reactivity of antisera with EV-D68 virus coated on the plate. Mouse antisera taken at 2 weeks after the last immunization were diluted 1:1000 and then used in this assay. c Neutralizing antibody titers against US/MO/14-18947 measured by the micro-neutralization assay. The antisera from mice immunized with control antigen did not exhibit any neutralization effect at 1:16 dilution (the lowest dilution tested) and were therefore denoted as a titer of eight for geometric mean titer (GMT) computation. Each symbol represents an individual mouse, and the solid line indicates the GMT of the group
Cross-neutralization activity of the pooled antisera against heterologous strains and viruses
One-day-old ICR mice born to dams immunized with EV-D68 VLPs or the control antigen were i.p. injected with 1.2 × 105 TCID50 of US/MO/14-18947. All infected mice were observed daily for a survival and b clinical score for 14 days. Clinical scores were graded as follows: 0, healthy; 1, lethargy and reduced mobility; 2, limb weakness; 3, limb paralysis; 4, death. The numbers of mice in each group were shown in brackets
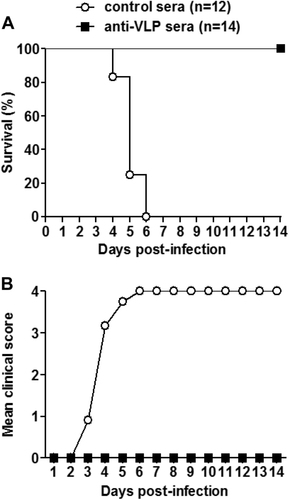
Groups of naive ICR mice (age <24 h) were i.p. injected with 20 µl of anti-VLP or control sera, and 1 day later i.p. inoculated with 1.2 × 105 TCID50 of US/MO/14-18947. Following challenge, mice were monitored daily for a survival and b clinical score for 14 days. Clinical scores were graded as described in the legend of Fig. . The numbers of mice in each group were shown in brackets