Figures & data
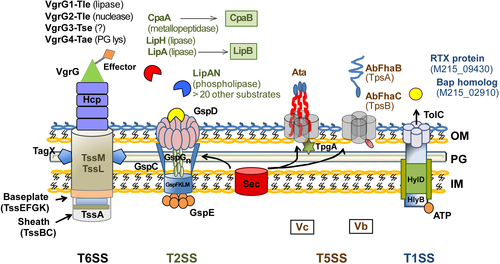
T6SS effectors are vgrG-associated and are encoded by genes that are in close proximity to each of the four vgrG genes identified in A. baumannii. The VgrG1-associated, VgrG2-associated, and VgrG4-associated effectors are type VI lipase effector (Tle), a type VI DNAse effector (Tde), and a peptidoglycan-targeting type VI amidase effector (Tae), respectively. The VgrG3 type VI effector (Tse) does not possess characteristic functional domains and is predicted to have a role in bacterial killing. A large number of T2SS effectors have been identified, including the verified lipolytic enzymes LipAN, LipH, and the chaperone-dependent LipA (chaperone LipB), in addition to chaperone-dependent CpaA metallopeptidase (chaperone CpaB). More than 20 other predicted T2SS effectors are yet to be verified as true substrates. Two T5SS subtypes have been identified in A. baumannii, the Acinetobacter trimeric autotransporter (Ata) and the two-partner secretion (TPS) system AbFhaB/FhaC, which plays a role in cellular adhesion. Both the T2SS and T5SS depend on the Sec machinery for the translocation of substrates across the inner cytoplasmic membrane (IM). In addition, A. baumannii possesses a homolog of the TolC-HlyB-D T1SS, which secretes a hemolysin-like RTX protein and a Bap-like protein important for biofilm formation