Figures & data
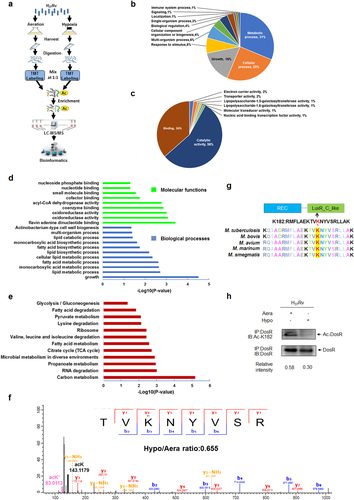
a Workflow of quantitative proteomics analysis of lysine acetylation in H37Rv under hypoxia. Mtb H37Rv was cultured under aeration or hypoxia in conical screw-capped Nephelo flasks with 20 mm side arms according to the Wayne model. Samples for aeration (Aera) and hypoxia (Hypo) cultures were harvested after 12 days. Proteins were extracted and digested by trypsin. After separate labeling with a tandem mass tag (TMT) 6-plex kit, peptides from Aera or Hypo were mixed 1:1, enriched with anti-acetyl lysine antibody beads and loaded for LC–MS/MS analysis. The resulting MS/MS data were processed for bioinformatics analysis. b, c Gene ontology functional classification of biological processes (b) and molecular functions (c) of 215 upregulated acetylated proteins under hypoxia. d Gene ontology enrichment analysis of biological processes and molecular functions of 215 upregulated acetylated proteins under hypoxia. The differently colored bars indicate the corrected p values for the enrichment of the annotations. e KEGG pathway enrichment analysis of 215 upregulated acetylated proteins under hypoxia. f Mass spectroscopy (MS) analysis identified K182 acetylation of DosR. g K182 in DosR is conserved among various mycobacteria species. The asterisk denotes the conserved lysine residue, and this result was analyzed by DNASTAR software. h Acetylation of DosRK182 in H37Rv WCL under hypoxia was downregulated. H37Rv were grown under aeration or hypoxia in 7H9 broth supplemented with 10% ADC for 3 days. DosR proteins were immunoprecipitated (IP) by the anti-DosR antibody. The acetylation level of DosR was determined by immunoblotting (IB) using an anti-DosR Ac-K182-specific polyclonal antibody. Western blots are representative of at least three independent experiments
Acetylation of six regulated proteins from Mtb hypoxia regulatory network
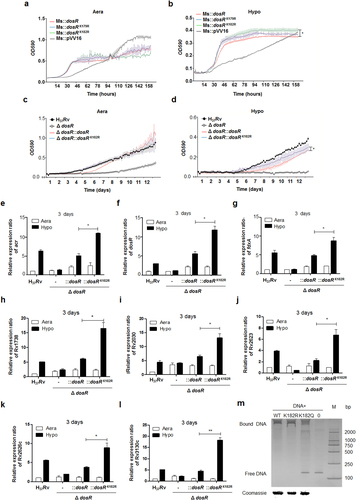
a, b Growth in aeration (a) or hypoxia (b) of M. smegmatis overexpressing DosR or a K-R mutant. Recombinant M. smegmatis overexpressing DosR or the K-R mutant were grown to mid-log phase; the growth curve was measured using a Bioscreen Growth Curve Instrument. M. smegmatis harboring pVV16 was used as a control. Hypoxic conditions were established by covering bacterial suspensions with paraffin oil. The optical density was measured at an absorbance of 590 nm every 2 h. Cultures were grown at 37 °C for 7 days. The results were combined (mean ± standard deviation (SD)) from two independent experiments, with each experiment performed in triplicate. A statistically significant difference was found between Ms::dosR and Ms::dosRK182R under hypoxic conditions, as analyzed by Student’s t-test (*P < 0.05). c, d Growth under aeration (c) or hypoxia (d) of H37Rv, the deletion mutant of dosR, and WT or K182R complement mutants. Growth curves were measured as described above, and cultures were grown at 37 °C for 14 days. A significant difference was also found between the WT complement strain and K182R complement mutants under hypoxia. *P < 0.05. Student’s t-test. e–l The K182R mutant promoted the transcription of the target genes acr (e), dosR (f), fdxA (g), Rv1738 (h), Rv2030 (i), Rv2623 (j), Rv2626c (k), and Rv3130c (l). Bacteria were cultured and harvested at day 3 to isolate total RNA. Relative transcriptional levels were determined using the 2−ΔΔCt method. The reference gene used was 16S rRNA. Values represent the mean ± SD from three independent experiments. *P < 0.05. ** P<0.01. Student’s t-test. m Deacetylation of K182 strengthened the DNA-binding activity of DosR. An electrophoretic mobility shift assay (EMSA) was used to test the binding of DosR and the derivative mutant proteins K182R and K182Q to the promoter region of acr. The EMSA result is representative of at least three independent experiments
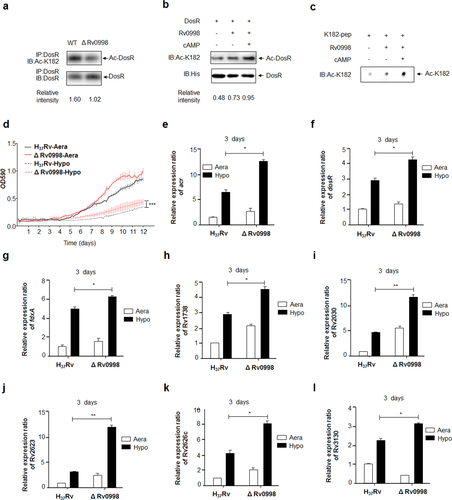
a Acetylation of DosRK182 in the Rv0998 deletion mutant of Mtb H37Rv was downregulated. DosR from the Rv0998 deletion mutant or the wild-type Mtb H37Rv was immunoprecipitated (IP) by an anti-DosR antibody. Acetylation levels were detected by immunoblotting (IB) with an anti-DosR Ac-K182-specific polyclonal antibody. Western blots were repeated at least three times—a representative blot is shown. b Rv0998 acetylates DosR K182 in vitro. DosR (2 μg) was incubated with or without Rv0998 (2 μg) or cAMP (200 μM). Acetylation levels were determined by immunoblotting. The western blot shown is representative of at least three independent experiments. c Rv0998 acetylates K182 peptides of DosR in vitro. K182 peptide (2 μg) was incubated, with or without Rv0998 (2 μg) or cAMP (200 μM). Acetylation levels were determined by dot blot. The dot blot shown is representative of at least three independent experiments. d Deletion of Rv0998 promoted the hypoxia response of Mtb. A growth curve was measured for the Rv0998 deletion mutant and wild-type Mtb H37Rv under aeration and hypoxia. e–l Deletion of Rv0998 promoted the transcription of acr (e), dosR (f), fdxA (g), Rv1738 (h), Rv2030 (i), Rv2623 (j), Rv2626c (k), and Rv3130c (l). Bacteria were cultured and harvested on day 3 to isolate total RNA. Relative transcriptional levels were determined using the 2−ΔΔCt method. The expression of tested genes was normalized to that of 16S rRNA. Values represent the mean ± SD from three independent experiments. *P < 0.05.** P<0.01. Student’s t-test
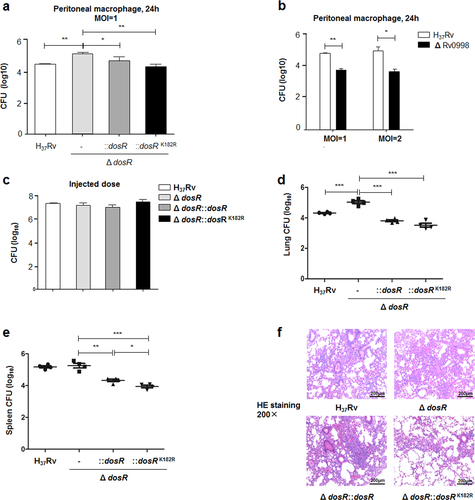
a Deacetylation of K182 inhibited the intracellular survival of Mtb. Primary peritoneal macrophages were harvested as described in Materials and Methods and infected at a multiplicity of infection (MOI) of 1 for 24 h. Infected cells were lysed, and the number of viable intracellular bacteria was determined by serial dilutions and plating on 7H10 agar plates. Values represent the mean ± SD of three independent experiments. b The deletion of Rv0998 inhibited the intracellular survival of Mtb. Peritoneal macrophages were infected at an MOI of 1 or 2 for 24 h. Bacterial colonies were counted to calculate intracellular survival efficiency. Values represent the mean ± SD of three independent experiments. c Doses of Mtb H37Rv, the deletion mutant of DosR, and WT or K182R complement mutants were injected into mice. The number of bacteria in the injected doses for the four bacterial groups was determined by serial dilutions and plating on 7H10 agar plates and was found to be similar for all groups. d, e Lung (d) and spleen (e) colony-forming units (CFUs) of C57Bl/6 mice infected for 28 days with Mtb H37Rv, the deletion mutant of dosR, and WT or K182R complement mutants. Female C57Bl/6 mice (6–8 weeks old) were challenged by intraperitoneal (i.p.) injection with 5 × 106 CFU in 100 μL PBS. The left side of the lung tissues from infected mice and the whole spleen were homogenized, diluted, and plated on 7H10 agar plates. Colonies were counted after 4 weeks. f Lung histopathology of C57Bl/6 mice infected with Mtb H37Rv, the deletion mutant of dosR, and WT or K182R complement mutants for 28 days. Lung halves of mice infected for 28 days were fixed and embedded in paraffin, and then 5 μm-thick sections were stained with hematoxylin and eosin (HE) by standard methods. The pathology was evaluated by pathologists in a blinded manner. Images are pseudocolored representations at ×200 magnification. Values represent the mean ± SD from two independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001. Student’s t-test