Figures & data
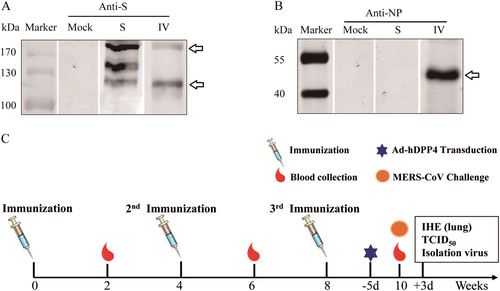
Western blot analyses of Middle East respiratory syndrome coronavirus (MERS-CoV) S and inactivated whole MERS-CoV(IV) vaccines using mouse anti-S (a) and anti-NP monoclonal antibodies (mAbs) (b). Schematic of the study (c)
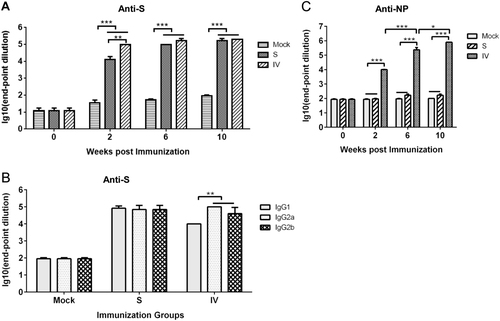
Anti-S (a) and anti-NP (b) specific IgGs were detected at 2, 6, and 10 weeks. S protein-specific antibody isotypes induced by vaccination after 10 weeks (c). Values are the means ± standard error of the mean (SEM). Significant values are defined by *P < 0.05, **P < 0.01 and ***P < 0.001
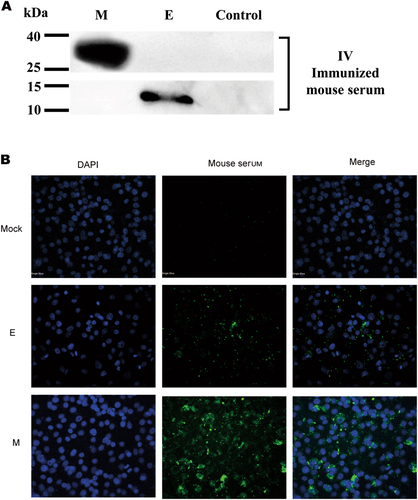
Samples were collected from 293 T cells that were transiently transfected with the pCAGGS-E (E), pCAGGS-M (M) or control plasmid pCAGGS at 24 h post-transfection and subjected to WB (a) or IFA (b) using IV-immunized mouse serum at a 1:400 dilution as the primary antibody
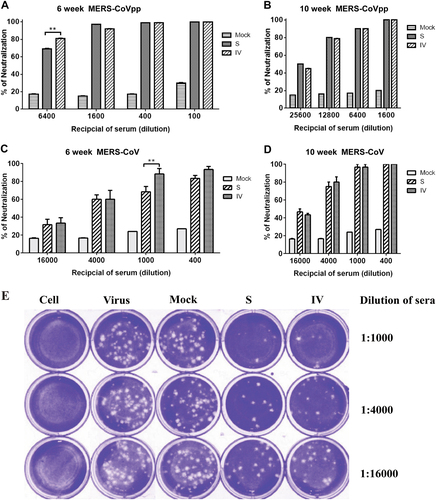
Neutralizing antibody titers against MERS-CoV pseudovirus particles. (a, b) and MERS-CoV (c, d) were determined by plaque reduction neutralization assays at 6 and 10 weeks. Representative results of the plaque reduction neutralization (PRNT) assay for the detection of neutralization activity in the sera of mice (e). Approximately 30 pfu of the virus stock (hCoV-EMC) was used to infect Vero cells in 12-well plates with or without heat-inactivated sera from immunized mice 2 weeks after the third immunization. PRNT50 was calculated after the plaques were counted. Significant values are defined by **p < 0.01.
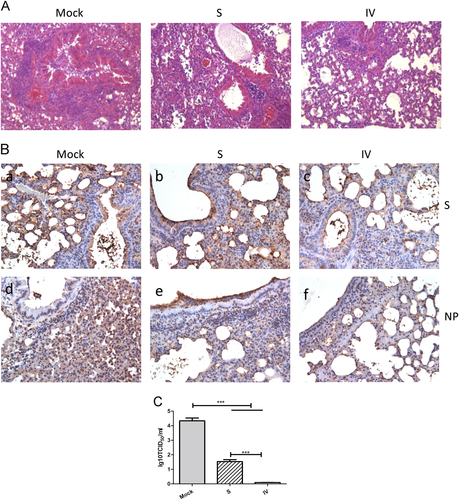
Representative results of hematoxylin-eosin (HE) staining (×400) in the lungs of mock-treated or immunized mice (a). Immunohistochemistry staining (×400) with anti-S and anti-NP mAbs (b). Lung virus titers 3 days after MERS-CoV challenge (c) as detected by virus isolation and titration at day 3 post-challenge. Values are the means ± SEM. Significant values are defined by ***P < 0.001