Figures & data
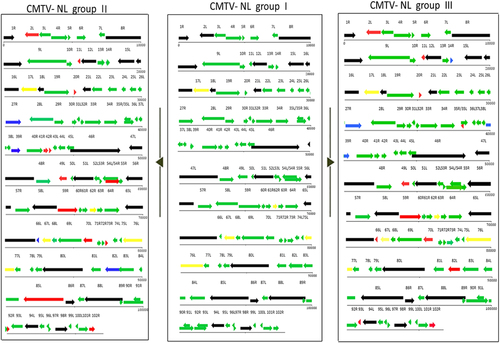
A representative layout of group I viruses is featured in the middle section to facilitate comparison to CMTV NL II (left) and III (right). Red arrows represent shortened ORFs with the white portion of the arrows representing the amount of lost nucleotides/amino acids. Blue arrows represent genes with ORFs larger than their counterparts in CMTV-NL group I. Black arrows represent core iridoviral proteins. Green arrows represent genes identical in all groups
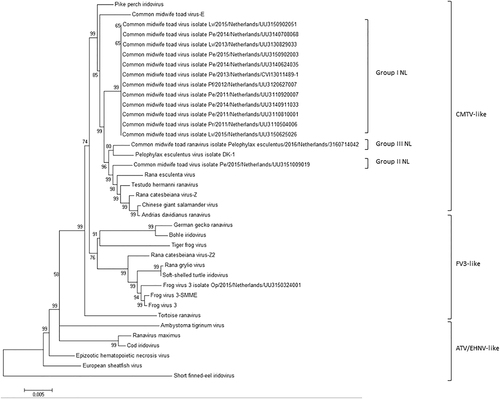
All Dutch ranaviruses cluster within the CMTV-like clade. Group II and III cluster closely with CMTV-like ranaviruses from China (ADRV, CGSIV), Italy (REV), Switzerland (THR), and Denmark (PEV-DEK1). Isolates and Genbank numbers used: common midwife toad ranavirus isolate Pelophylax kl.esculentus/2013/NL (KP056312), common midwife toad ranavirus isolate Mesotriton alpestris/2008/E (JQ231222), Rana esculenta virus isolate REV 282/I02 (MF538628), Pelophylax esculentus virus isolate PEV_DK1 (MF538627), Rana catesbeiana virus 1 isolate RCV-Z (MF187210), R. catesbeiana virus 2 isolate RCV2-Z2 (MF187209), Frog virus 3 (AY548484), Frog virus 3 isolate SSME (KJ175144), tortoise ranavirus isolate 1 (882/96) (KP266743), Testudo hermanni ranavirus isolate CH8/96 (KP266741), German gecko ranavirus isolate 2000/99 (KP266742), tiger frog virus (AF389451), soft shelled turtle iridovirus (EU627010), European sheathfish virus (JQ724856), Epizootic hematopoietic necrosis virus (FJ433873), Ambystoma tigrinum stebbensi virus (AY150217), Andrias davidianus ranavirus isolate 1201 (KC865735), Chinese giant salamander iridovirus, isolate CGSIV-HN1104, (KF512820), Rana grylio ranavirus (JQ654586), pike perch iridovirus isolate SLU 144001 (KX574341), Bohle iridovirus isolate BIV-ME 93/95, cod iridovirus isolate GAM14001 (KX57432), ranavirus maximus isolate SMA15001 (N_C030842), and short-finned eel ranavirus isolate ANGA14001 (KX353311)
Comparison of individual genes between CMTV-NL groups I, II, and III
Analysis of ranavirus repetitive regions
The figure depicts the average titer of each virus group and time point from three independent experiments. Error bars represent standard deviation
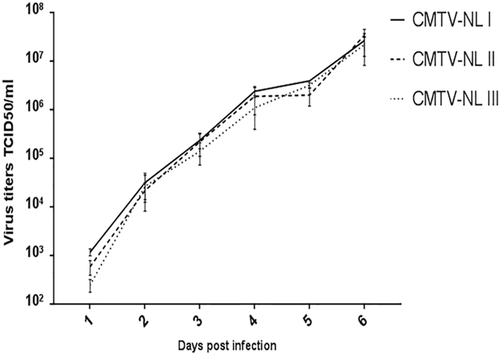
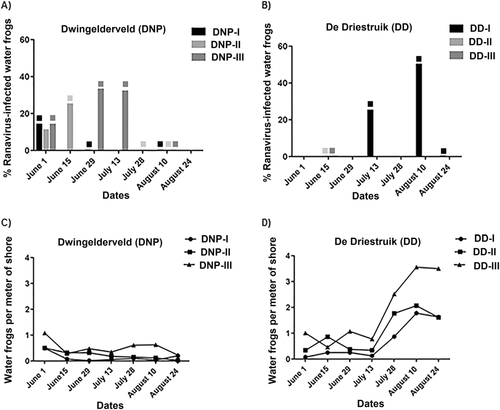
Fig. 4 Proportion of ranavirus in swabbed specimens and adult water frog abundance (a, b) Ranavirus was detected from swabs and percentage of positive animals at each site is given. Water samples positive for ranavirus are indicated by blocks matching the color of the waterbody; (c, d) The ratio of water frog per shore meter is depicted for each visit for all waterbodies. No water frogs could be found for swabbing at DNP-I in visits June 15, June 29, July 13, July 28, and August 24, or in DNP-II from visit July 28 onwards
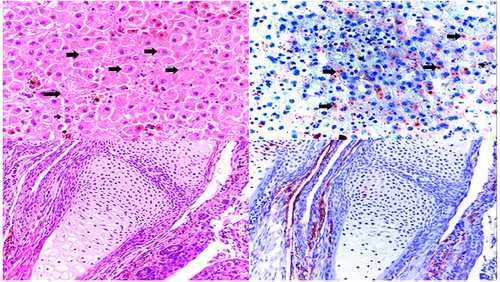
Top panel: (left) Hematoxylin/Eosin staining of a liver section from an affected water frog adult from DNP with intracytoplasmic basophilic inclusions (black arrows) and (right) immunohistochemical staining of a serial section in which intracytoplasmic inclusions present with marked immunolabelling confirming active viral replication. Lower panel: (left) hematoxylin/eosin staining of forelimb from affected water frog larva with no apparent microscopic lesion; (right) immunohistochemical staining of a serial section, in which positive immunolabelling is present in the periarticular muscle and connective tissue