Figures & data
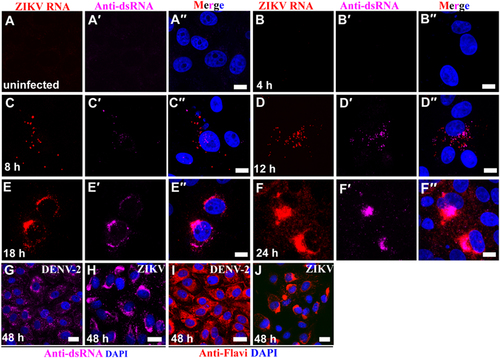
(a–a′′) Vero 76 control cells (uninfected cells). (b–f′′) Vero 76 cells were infected with ZIKV African MR 766 strain and IFA/FISH-stained using a probe targeting the ZIKV genome (red) and J2 antibody against dsRNA (magenta) at the indicated time points. (g–j) Location of ZIKV perinuclear replication factories was compared with even distribution of DENV-2 in the cytoplasm by staining of antibody J2 against dsRNA (magenta) and antibody against flaviviruses (red). Nuclei were stained with DAPI (blue). Scale bar, 10 µm (a–f′′) and 20 µm (g–j)
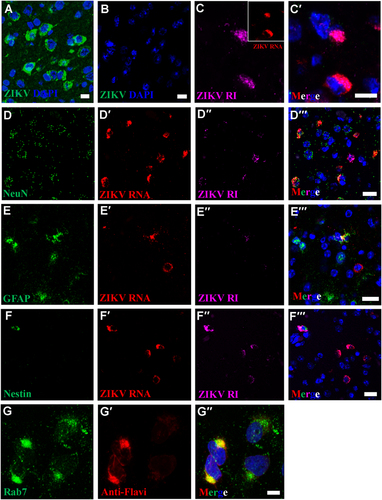
IFA using anti-ZIKV E antibody reveals perinuclear ZIKV replication factories in infected (a), but not in uninfected mouse brains (b). c–c′ Perinuclear ZIKV replication factories were revealed by multiplex FISH detecting ZIKV genomic (inset, red) or antigenomic RNA (magenta). (d–f′′) Multiplex FISH detection of ZIKV replication factories in neurons (Rbfox3/NeuN), glial cells (GFAP), and neuroectodermal stem cells (Nes). (g–g′′) Colocalization of ZIKV particles and endosomes were revealed by IFA using anti-flavivirus antibody (red) and anti-Rab7 antibody (green). Nuclei were stained with DAPI (blue). Scale bar, 10 µm (a–c′, g–g′) and 20 µm (d–f′)
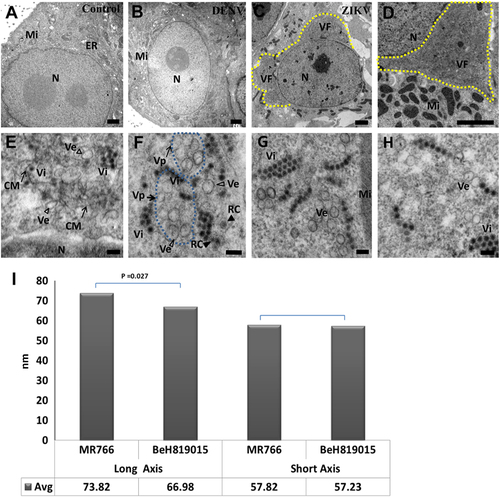
ZIKV-infected cells were analyzed 48 h post-exposure and analyzed by TEM. (a) In the perinuclear region of uninfected Vero 76 cell, endoplasmic reticulum (ER) membranes are organized in orderly fashion. Mi, mitochondria; N, nuclei. (b) In DENV-2-infected Vero 76 cells, convoluted ER membranes are evident, but visible perinuclear replication factories are lacking. (c) Perinuclear ZIKV replication factory (VF, yellow dashed line) in the degenerated rough ER. (d) Multiple mitochondria (Mi) in proximity to the ZIKV replication factory. Large numbers of ZIKV particles are visible in both panels (tiny, dark pin dots). (e, f) Convoluted membranes (CM, black arrows) and vesicular structures (empty arrowheads) are located within ZIKV replication factories. Aggregated vesicles (Ve) were wrapped in a packet (Vp, black arrows, blue dashed line). Note the likely presence of replication centers (RC, solid arrow heads) in two of the vesicles and newly assembled clusters of virions (Vi). Scale bar, 2 µm (a–d), 500 nm (e), and 100 nm (f–h). (g, h) Representative images of induced vesicles inside cells infected with ZIKV MR766 (e) and BeH819015 strains (f). (i) Measurement of vesicle sizes induced by ZIKV MR766 (n = 162) and BeH819015 strains (n = 44)
(a) ZIKV particle cell entry. Note the thickening of the membrane and formation of a clathrin-coated pit. (b) A ZIKV particle enters the host cell via formation of a clathrin-coated vesicle. (c) The ZIKV particles (arrow heads) are leaving early endosomal (EE) vacuoles. (d) A late endosome (multivesicular body [MVB]) with assembled virus particles. (e) Replication centers (RC) inside vesicles wrapped in packets (Vp, black dashed arrows). (f) ZIKV paracrystalline array in the ER lumen. Note incompletely assembled particles (partial or empty: empty arrowhead) next to mature particles (solid arrowhead). (g) An MVB sac containing vesicles about to be abscised from the neck of the cytoplasmic membrane. (h) Virions (Vi) egressing from the MVB sac. Inset shows virons at high magnification. (I) Mature virus particle budding from the cytoplasmic membrane. (j) Released virus particles in the extracellular space. Scale bar, 100 nm
![Fig. 4 Uptake, replication, and egress of ZIKV from inside the cells.(a) ZIKV particle cell entry. Note the thickening of the membrane and formation of a clathrin-coated pit. (b) A ZIKV particle enters the host cell via formation of a clathrin-coated vesicle. (c) The ZIKV particles (arrow heads) are leaving early endosomal (EE) vacuoles. (d) A late endosome (multivesicular body [MVB]) with assembled virus particles. (e) Replication centers (RC) inside vesicles wrapped in packets (Vp, black dashed arrows). (f) ZIKV paracrystalline array in the ER lumen. Note incompletely assembled particles (partial or empty: empty arrowhead) next to mature particles (solid arrowhead). (g) An MVB sac containing vesicles about to be abscised from the neck of the cytoplasmic membrane. (h) Virions (Vi) egressing from the MVB sac. Inset shows virons at high magnification. (I) Mature virus particle budding from the cytoplasmic membrane. (j) Released virus particles in the extracellular space. Scale bar, 100 nm](/cms/asset/684b1732-649d-4d81-a707-377cd1619033/temi_a_12039978_f0004_ob.jpg)
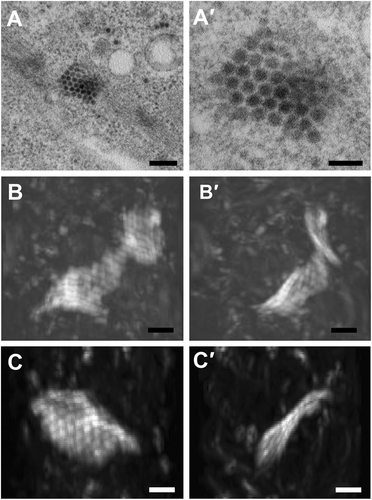
(a–a′) ZIKV paracrystalline (PC) array structure at low and high magnifications of transmission electron micrographs (TEM). (b–b′) Reconstructed 3D images from focused ion beam scanning electron microscopy (FIB-SEM) at two different angels reveal a wavy sheet-like array of a single layer of ZIKV particles. (c–c′) Another sheet-like ZIKV particle array structure at two different angles. The clockwise rotation angles of (b′) and (c′) relative to (b) and (c) are 110 degrees around the vertical axis. Scale bar, 200 nm (a) and 100 nm (a′–c′)