Figures & data
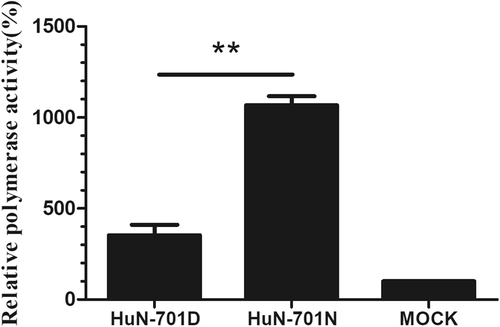
293T cells were transfected with a pFluc plasmid expressing negative-sense virus-like RNA and encoding firefly luciferase (Fluc) and a pRluc plasmid expressing the Renilla luciferase gene (Rluc) as an internal control (Promega). The 293T cells were also co-transfected with plasmids expressing the wild-type (701D) and mutated (701 N) PB2, PB1, NP, and PA segments derived from the A/Hunan/42443/2015(H1N1) viruses. After being cultured at 37 °C for 24 h, cell lysates were used to measure the Fluc and Rluc activity levels. Mock transfection with the two reporter constructs only was set to 100%. The means ± SD of triplicate experiments are shown. **P < 0.01
Eight- to ten-week-old female C57BL/6 mice (n = 5/group) were inoculated intranasally with 101 (a), 102 (b), 103 (c), 104 (d), 105 (e), and 106 TCID50 (f) of the recombinant viruses. Mice receiving PBS were used as controls. Weight loss was monitored for 14 days
Eight- to ten-week-old female C57BL/6 mice (n = 5/group) were inoculated intranasally with 101 (a), 102 (b), 103 (c), 104 (d), 105 (e), 106 TCID50 (f) of the recombinant viruses. Mice receiving PBS were used as controls. Survival was monitored for 14 days
Seroconversions of mice inoculated with recombinant virusesa
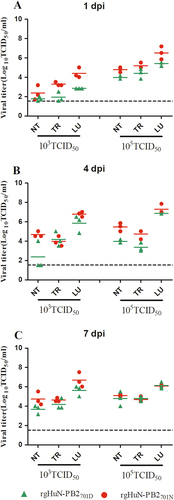
Eight- to ten-week-old female C57BL/6 mice (n = 3/time-point) were inoculated intranasally with 103 TCID50 (left) or 105 TCID50 (right) of the recombinant viruses. Animals were euthanized at 1 (a), 4 (b), and 7 (c) dpi. Viral titers in the tissues were determined on MDCK cells. NT Nasal turbinate, TR trachea, LU lung
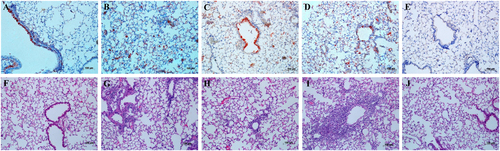
Eight- to ten-week-old female C57BL/6 mice (n = 3/time-point) were inoculated intranasally with 103 TCID50 of the rgHuN-PB2701D viruses (a, f) and rgHuN-PB2701N viruses (c, h) or 105 TCID50 of the rgHuN-PB2701D viruses (b, g) and rgHuN-PB2701N viruses (d, i). Mice receiving PBS (e, j) were used as controls. Mice were killed at 4 dpi, and the lung lobes were used for NP antigen staining (upper panel) and hematoxylin and eosin (H&E) staining (lower panel)
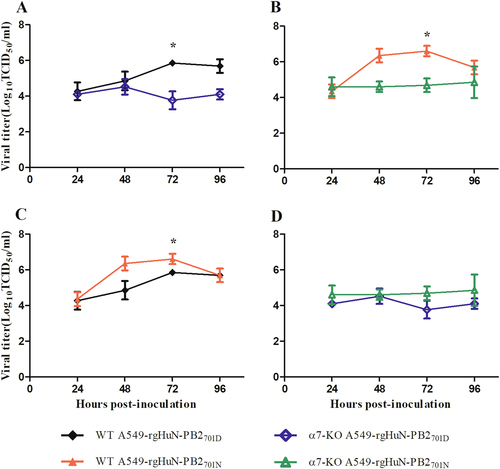
Cells were inoculated with the recombinant viruses at 37 °C, and culture supernatants were harvested at 0, 24, 48, 72 and 96 h post-inoculation (hpi). Virus titers were determined on MDCK cells. a Growth curves of the rgHuN-PB2701D viruses in WT (black, filled diamonds) and α7-KO (blue diamonds) A549 cells; b Growth curves of the rgHuN-PB2701N viruses in WT (red, filled triangles) and α7-KO (green triangles) A549 cells; c Growth curves of the rgHuN-PB2701D viruses (black, filled diamonds) and the rgHuN-PB2701N viruses (red, filled triangles) in WT A549 cells; d Growth curves of the rgHuN-PB2701D viruses (blue diamonds) and the rgHuN-PB2701N viruses (green triangles) in α7-KO A549 cells. The results are presented as the means ± SD of three repeated experiments. *P < 0.05
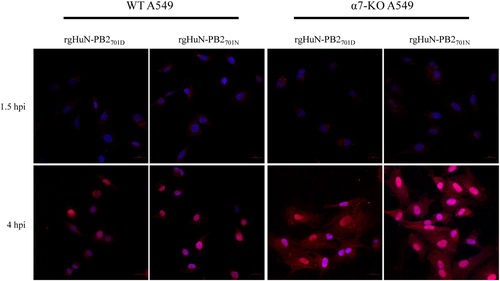
WT and α7-KO A549 cells were infected with recombinant viruses at 37 °C and were fixed at indicated time points (1.5 hpi early in the life cycle and 4 hpi in the middle of the life cycle). Cells were then immunostained with an antibody against NP and an Alexa Fluor 594-conjugated secondary antibody. Cell nuclei were stained with DAPI