Figures & data
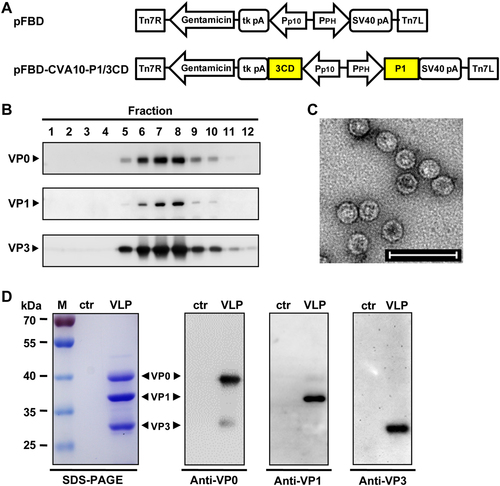
a Diagrams of plasmids pFBD and pFBD-CVA10-P1/3CD. Tn7R and Tn7L, right and left elements of Tn7 transposon; Gentamicin, gentamicin resistance gene; tk pA, herpes simplex virus thymidine kinase (tk) polyadenylation signal; Pp10, Autographa californica multiple nuclear polyhedrosis virus (AcMNPV) p10 promoter; PPH, AcMNPV polyhedrin promoter; SV40 pA, SV40 polyadenylation signal. b Sucrose gradient sedimentation analysis. Lysates from Sf9 cells infected with baculovirus Bac-CVA10-P1/3CD were subjected to 10–50% sucrose gradient centrifugation. Twelve fractions were collected from the top of the gradient, followed by western blotting analysis using polyclonal antibodies against CVA10 VP0, VP1, and VP3 proteins. c Transmission electron microscopy imaging of CVA10-VLP. Scale bar = 100 nm. d SDS-PAGE and western blotting analysis of the purified CVA10-VLP sample. Lane M, protein marker; ctr, control antigen produced from uninfected Sf9 cells; and VLP, purified CVA10-VLP
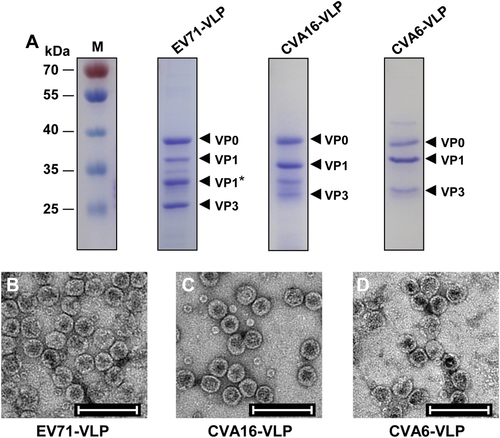
a SDS-PAGE analysis of purified EV71-VLP, CVA16-VLP, and CVA6-VLP. Lane M, protein marker. b–d Electron microscopy micrographs of purified b EV71-VLP, c CVA16-VLP, and d CVA6-VLP. Scale bar = 100 nm
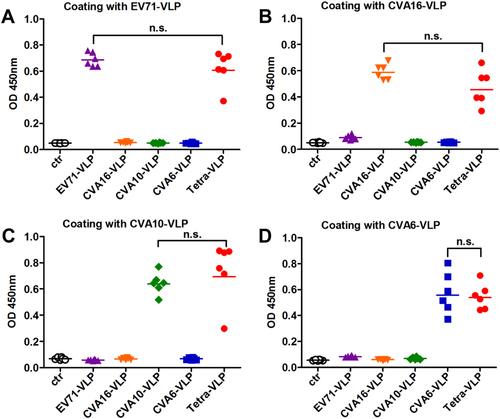
Groups of six BALB/c mice were immunized with the control antigen (ctr), EV71-VLP, CVA16-VLP, CVA10-VLP, CVA6-VLP, or the tetravalent VLP (VLPs of EV71, CVA16, CVA10, and CVA6; termed Tetra-VLP). Serum samples were harvested from each mouse two weeks after final immunization, diluted 1:1000, and then analyzed for antigen-specific IgG antibodies by ELIZA using a EV71-VLP, b CVA16-VLP, c CVA10-VLP, and d CVA6-VLP as capture antigens. Each symbol represents a mouse, and the solid line indicates the geometric mean value of the group. Statistical significance was determined by a two-tailed Student’s t-test and is indicated as follows: n.s., no significant difference (P ≥ 0.05); *P < 0.05; **P < 0.01; and ***P < 0.001
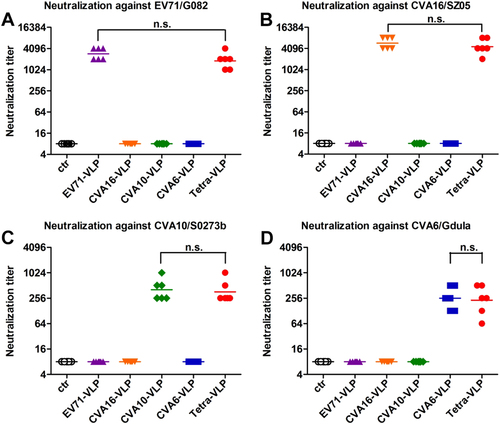
Mouse antisera obtained two weeks after the last immunization dose were subjected to micro-neutralization assays to measure neutralizing antibody titers against a EV71/G082, b CVA16/SZ05, c CVA10/S0273b, and d CVA6/Gdula. For geometric mean titer (GMT) calculation, a titer of 8 was assigned to serum samples with no detectable neutralizing antibodies at the lowest dilution tested (1:16). Each symbol represents a mouse, and the solid line denotes the geometric mean value of each group. Statistical significance is indicated as follows: n.s., no significant difference (P ≥ 0.05); *P < 0.05; **P < 0.01; and ***P < 0.001
Neutralization activity of pooled antisera against a panel of enteroviruses
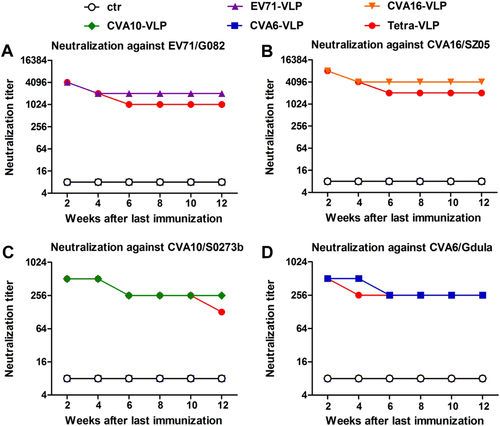
Antisera were taken from each immunized mouse every two weeks after the last vaccination dose and pooled for each group. The resulting antisera pools were subjected to micro-neutralization assays to detect neutralizing antibody titers against a EV71/G082, b CVA16/SZ05, c CVA10/S0273b, and d CVA6/Gdula. Serum samples that did not confer any neutralization at the starting dilution of 1:16 were assigned a titer of 8
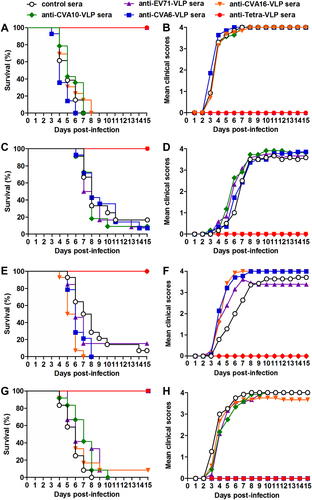
Groups of six-day-old ICR mice (n = 11–14 mice/group) were injected i.p. with pooled anti-EV71-VLP, anti-CVA16-VLP, anti-CVA10-VLP, anti-CVA6-VLP, anti-Tetra-VLP, or control sera. One day later, the suckling mice were i.p. challenged with a, b EV71/MAV-W, c, d CVA16/MAV, e, f CVA10/S0148b, or g, h CVA6/S0087b. After challenge, all mice were monitored daily for (a, c, e, g) survival and (b, d, f, h) clinical score for 15 days. Clinical scores were graded as follows: 0, healthy; 1, reduced mobility; 2, limb weakness; 3, paralysis; and 4, death
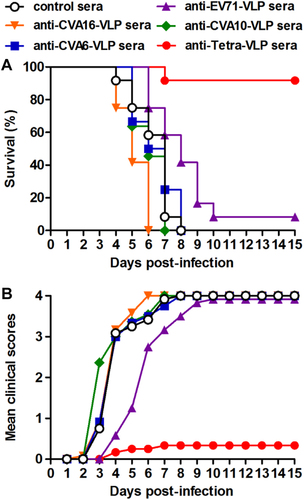
Groups of six-day-old ICR mice (n = 11–12/group) were i.p. administered with pooled anti-EV71-VLP, anti-CVA16-VLP, anti-CVA10-VLP, anti-CVA6-VLP, anti-Tetra-VLP, or control sera, followed by simultaneous inoculation one day later with EV71/MAV-W, CVA16/MAV, CVA10/S0148b, and CVA6/S0087b. a Survival and b clinical score were monitored daily for 15 days following challenge. Clinical scores were graded as described in the legend of Figure