Figures & data
a Seven-week-old Balb/c female mice were intramuscularly immunized with 5 × 108 vp rAd2-ZGP, 5 × 109 vp rAd2-ZGP, 2 µg ZGP, 20 µg ZGP, 2 µg ZVLPs, or 20 µg ZVLPs. Mice injected with PBS, 5 × 108 vp or 5 × 109 vp rAd2-Empty were used as control groups. b Three weeks after immunization, serum samples were collected and subjected to ELISA analysis of IgG antibodies that bind to ZEBOV GP and SEBOV GP. The titers were calculated as reciprocal endpoints. A cutoff value for a positive result was calculated as the mean optical density (at a 1:100 dilution) for the control serum sample plus 3 SDs. c Three weeks after immunization, the serum samples were measured for the neutralizing activities to ZEBOV GP pseudo-typed lentivirus or SEBOV GP pseudo-typed lentivirus. Pseudo-typed lentiviruses at 100 TCID50 were incubated with 8 serial twofold dilutions of serum samples from each group and infected into Huh-7 cells. The neutralizing activity was measured as the decrease of luciferase expression relative to negative control sera. The IC50 was calculated by the dose–response inhibition function in GraphPad Prism 7.00. Data are presented as the mean ± SD (n = 5). d Mice immunized with higher dosage groups were sacrificed 3 weeks after immunization. Splenocytes were isolated and stimulated with a peptide pool derived from ZEBOV GP. IFN-γ+ SFCs were assessed with an ELISpot assay and imaged with an ELISpot reader. Data are shown as the number of SFCs in one million splenocytes. e CD8+ T cells secreting IFN-γ were determined with an ICS assay after stimulation with a peptide pool derived from ZEBOV GP. Data are shown as the mean ± SD (n = 5). f Splenocytes were stimulated with either ZEBOV GP or SEBOV GP. IFN-γ+ SFCs were assessed with an ELISpot assay. Data are shown as the number of SFCs in one million splenocytes. Comparisons between groups were performed by one-way ANOVA, comparisons of the neutralizing antibody titers between low- and high-dose immunizations in the rAd2-ZGP group were performed by Student’s t test, and a P-value < 0.05 was considered statistically significant. *, P < 0.05; **, P < 0.01; ***, P < 0.001
a Seven-week-old Balb/c female mice were intramuscularly injected with 5 × 108 vp rAd2-ZGP, 2 µg ZGP, or 2 µg ZVLPs. Six weeks later, mice received a booster immunization of the same vaccine. Mice injected with PBS or 5 × 108 vp rAd2-Empty were used as control groups. b Three weeks after each immunization, serum samples were collected and subjected to ELISA analysis of IgG antibodies that bind to ZEBOV GP and SEBOV GP. The titers were calculated as reciprocal endpoints. A cutoff value for a positive result was calculated as the mean optical density (at a 1:100 dilution) for the control serum sample plus 3 SDs. c Three weeks after each immunization, serum samples were measured for neutralizing activities to the ZEBOV GP pseudo-typed lentivirus or SEBOV GP pseudo-typed lentivirus. Pseudo-typed lentiviruses at 100 TCID50 were incubated with 8 serial twofold dilutions of serum samples from each group and infected into Huh-7 cells. The neutralizing activity was measured as the decrease of luciferase expression relative to negative sera. The IC50 was calculated by the dose–response inhibition function in GraphPad Prism 7.00. Data are presented as the mean ± SD (n = 5). d Mice were sacrificed 3 weeks after the booster immunization. Splenocytes were isolated and stimulated with a peptide pool derived from ZEBOV GP. IFN-γ+ SFCs were assessed with an ELISpot assay and imaged with an ELISpot reader. Data are shown as the number of SFCs in one million splenocytes. e CD8+ T cells secreting IFN-γ were determined using an ICS assay after stimulation with a peptide pool derived from ZEBOV GP. Data are shown as the mean ± SD (n = 5). f Splenocytes were stimulated with either ZEBOV GP or SEBOV GP. IFN-γ+ SFCs were assessed with an ELISpot assay. Data are shown as the number of SFCs in one million splenocytes. Comparisons between groups were performed by one-way ANOVA; comparisons of the neutralizing antibody titers between prime and booster immunizations in the rAd2-ZGP group were performed by Student’s t test. A P-value < 0.05 was considered statistically significant. *, P < 0.05; **, P < 0.01; ***, P < 0.001
a Chinese rhesus macaques were divided into four groups and immunized intramuscularly with either 1 × 1011 vp rAd2-ZGP, 400 µg ZGP, 400 µg ZVLPs, or PBS. After 4 weeks, macaques received a booster immunization of the same vaccine. b At 0, 2, 4, and 6 weeks after the first immunization, serum samples were collected and subjected to ELISA analysis of IgG antibodies to ZEBOV GP and SEBOV GP. The titers were calculated as reciprocal endpoints. Control serum samples were run each time the assay was performed. A cutoff value for a positive result was calculated as the mean optical density (at a 1:100 dilution) for the control serum sample plus 3 SDs. c Serum samples were measured for the neutralizing activities to ZEBOV GP pseudo-typed lentivirus or SEBOV GP pseudo-typed lentivirus. Pseudo-typed lentiviruses at 100 TCID50 were incubated with 8 serial twofold dilutions of serum samples from each group and infected into Huh-7 cells. The neutralizing activity was measured as the decrease of luciferase expression relative to negative sera. The IC50 was calculated by the dose–response inhibition function in GraphPad Prism 7.00. Data are presented as the mean ± SD (n = 4). d PBMCs were collected 2 weeks after the first and second immunizations and were stimulated with a peptide pool derived from ZEBOV GP. IFN-γ+ SFCs were assessed with an ELISpot assay. Data are shown as the number of SFCs in one million PBMCs. e PBMCs were collected at 2 weeks after each immunization. PBMCs were stimulated with either ZEBOV GP or SEBOV GP. IFN-γ+ SFCs were assessed with an ELISpot assay. Data are shown as the number of SFCs in one million PBMCs. f Qualitative profiles of ZEBOV GP-specific CD8+ T cell responses in PBMCs at 2 weeks after a booster immunization. GP-specific CD8+ T cells secreting IFN-γ+, IL-2, and TNF-α were determined with ICS analysis. Data are presented as the mean ± SD (n = 4). Comparisons between groups were performed by one-way ANOVA; comparisons of the neutralizing antibody titers between prime and booster immunizations in the rAd2-ZGP group were performed by Student’s t test. A P-value < 0.05 was considered statistically significant. *, P < 0.05; **, P < 0.01; ***, P < 0.001
Reciprocal ELISA titers and neutralizing antibody titers 2 weeks after each immunization in rhesus macaques
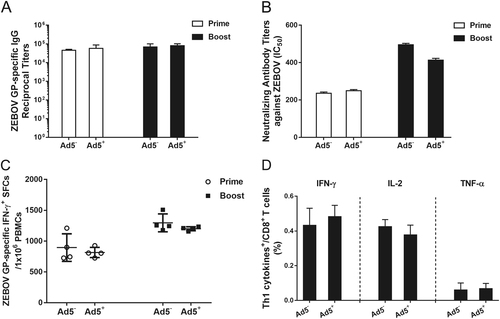
Chinese rhesus macaques seropositive for Ad5 neutralizing antibodies were immunized intramuscularly with 1 × 1011 vp rAd2-ZGP. After 4 weeks, these macaques received a booster immunization of the same vaccine. a Serum samples were collected and subjected to ELISA analysis of IgG antibodies that bind to ZEBOV GP. The titers were calculated as reciprocal endpoints. Control serum samples were run every time the assay was performed. A cutoff value for a positive result was calculated as the mean optical density (at a 1:100 dilution) for the control serum sample plus 3 SDs. b Serum samples were measured for the neutralizing activities to ZEBOV GP pseudo-typed lentivirus. Pseudo-typed lentiviruses at 100 TCID50 were incubated with 8 serial twofold dilutions of serum samples from each group and infected into Huh-7 cells. The neutralizing activity was measured as the decrease of luciferase expression relative to negative sera. The IC50 was calculated by the dose–response inhibition function in GraphPad Prism 7.00. Data are presented as the mean ± SD (n = 4). c PBMCs were collected 2 weeks after each immunization. PBMCs were stimulated with a peptide pool derived from ZEBOV GP. IFN-γ+ SFCs were assessed with an ELISpot assay. Data are shown as the number of SFCs in one million PBMCs. Data are presented as the mean ± SD (n = 4). d Qualitative profiles of ZEBOV GP-specific CD8+ T cell responses in PBMCs 2 weeks after a booster immunization. GP-specific CD8+ T cells secreting IFN-γ, IL-2, and TNF-α were determined with ICS analysis. Data are presented as the mean ± SD (n = 4)